ABSTRACT
When investigating the origins of targeted toxins (a drug, therapy, or scientific tool directed to unique extracellular target), an appropriate place to begin is with the Nobel Prize-winning work of Paul Ehrlich and his concept of the “magic bullet.” Over 100 years later, the use of targeted toxins to perform molecular neurosurgery has become a vital practice that allows researchers to observe changes in organisms after eliminating a neuronal population. A prime example of this practice is the specific targeting of cholinergic neurons in the basal forebrain to mimic Alzheimer’s Disease (AD). The research tool designed for this purpose is 192-IgG-Saporin, an antibody conjugated to the ribosome-inactivating protein (RIP) Saporin. Researchers have used this targeted toxin for over 30 years. A 2019 publication by Verkhratsky reviews AD models and states this is the only lesion model that specifically targets cholinergic neurons.[1]
In 1983, during a quest to find the optimal payload for a targeted toxin, Fiorenzo Stirpe and colleagues discovered Saporin, a plant protein isolated from the common soapwort plant Saponaria officinalis. Unlike ricin and abrin, Saporin does not have a binding chain and cannot enter a cell on its own.
Scientists have devised new ways to use Saporin to advance their research and drug development activities. Just a few examples include:
- A novel suicide gene therapy approach that uses a vector encoding a double-stranded DNA aptamer to deliver the gene encoding Saporin,
- Delivery of Saporin encapsulated in a nanotechnology system for development of cancer treatments,
- A deeper understanding of the difference between pain and itch and the relevant pathways, and
- Development of stable epilepsy animal model that is used for screen specific treatments that will lead to micro-methods to eliminate the disease.
This review will focus on Saporin as the payload delivered to cells. Targeted toxins (typically targeted by an antibody or peptide chemically linked or genetically fused) provide robust tools for neuroscience where ablation of specific neuronal populations is used to study behavior and function. Saporin is an ideal molecule because of its extreme resistance to high temperatures and denaturation, retention of catalytic activity after conjugation, and lack of a binding chain to allow entrance to the cytoplasm of cells on its own. As a result, it is one of the most studied RIPs used for its vigorousness, potency, safety, and ease of use in the laboratory. The information presented will shed light on the history of Saporin, current applications, and what the future holds for this protein in the neuroscience field.
Discovery/Function of Saporin
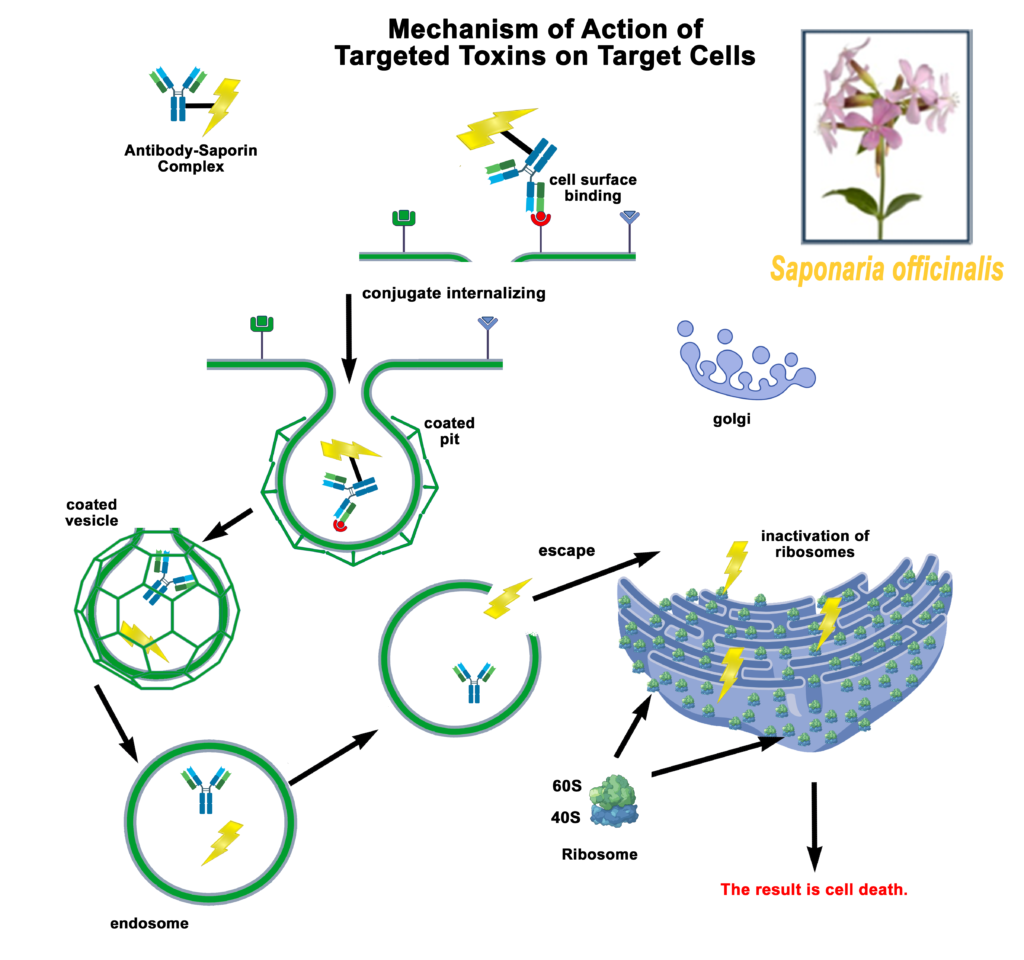
Saporin, a plant toxin isolated from the common soapwort plant Saponaria officinalis, was first characterized by researchers at the University of Bologna led by Fiorenzo Stirpe. [2] It was identified as a good-sized chromatography peak of the soluble extract of the plant, peak 6 or SO-6 with high activity and stability. It was classified as belonging to the family of single chain Ribosome-Inactivity-Proteins (RIPs). The mechanism of action for Saporin is to inhibit protein synthesis by cleaving the N-glycosidic bond of a specific adenine in 28 S rRNA which results in cell death. [2,3,4,5] Without a cell-binding chain, [6] Saporin has no method of internalization without a targeting agent to escort it into a cell. RIPs have long been thought of as potentially perfect parts for treatment of human tumors, tools to make Ehrlich’s Magic Bullet [7] and have been investigated extensively for use as therapeutic tools for cancer therapy. [8,9,10,11]
Stirpe later collaborated with a group led by Phil Thorpe to publish activity of a saporin immunotoxin in a lymphoma model. [12] From that project came a very telling comment, the authors stated that “Ricin A-chain coupled to OX7 antibody was one hundredfold to on thousandfold less effective than OX7-saporin as an antitumor agent in vivo.” During the years, a saporin targeted toxin has been used many times for the treatment of human disease, [13,14,15] but has not yet been approved as an anti-cancer drug component. Efforts to produce an approved saporin as a cancer drug continue, as seen recently. [16] The first use of a saporin toxin in the nervous system was presented at Art Frankel’s Second International Symposium on Immunotoxins in June of 1991. The use of fibroblast growth factor-saporin (FGF-SAP) in the inhibition of restenosis after antioplasty was an exciting use, but the organizer of the session, Ronald G. Wiley was more interested in a brief account of injection of FGF-SAP into the left corpus callosum and the effect on the opposite side. After the session, Wiley proposed the use of saporin conjugated to an antibody to the low affinity neuron receptor which was selectively expressed on neurons of the rat cholinergic neurons of the basal forebrain. This resulted in the removal of these rat neurons and the following publication was greeted warmly by the neuroscience community that had long desired a specific toxin for those cells as a model for Alzheimer’s Disease. [17]
Immunotoxin 192-IgG-SAP (192-Saporin)
192-IgG-SAP is a chemical conjugate of the mouse monoclonal antibody 192-IgG that recognizes low affinity neurotrophin receptor and the ribosome-inactivating protein, Saporin. This anti-neuronal immunotoxin permanently eliminates cholinergic neurons of the basal forebrain (CBF), medial septum and diagonal band of Broca, which provide critical inputs to the hippocampus. [18] 192-IgG-SAP also targets neurons of nucleus basalis of Meynert and Purkinje neurons of the cerebellum, but not projections to the amygdala.
192-IgG-SAP was first used for neuronal depletion in 1991; [17] four years later, the first Alzheimer’s disease (AD) rat model was developed by intraventricular injection.[19] Currently, 192-IgG-SAP is used to create animal models for the study of behavior, neuronal loss, plasticity of other systems in response to loss, replacement therapy, and drug effects and dependence.
The effect of CBF neuronal loss is observed in neurodegenerative diseases such as AD and dementia. These losses are linked to decreased neurogenesis in the hippocampus, a region involved in learning and memory. [20] Selective lesion of CBF neurons significantly impairs hippocampus-dependent memory function in rat. [18] This correlates to loss of cholinergic projections from the medial septum area to the hippocampus that are significantly reduced in AD patients, which explains the memory loss. This results in a rat model with aspects of human AD.
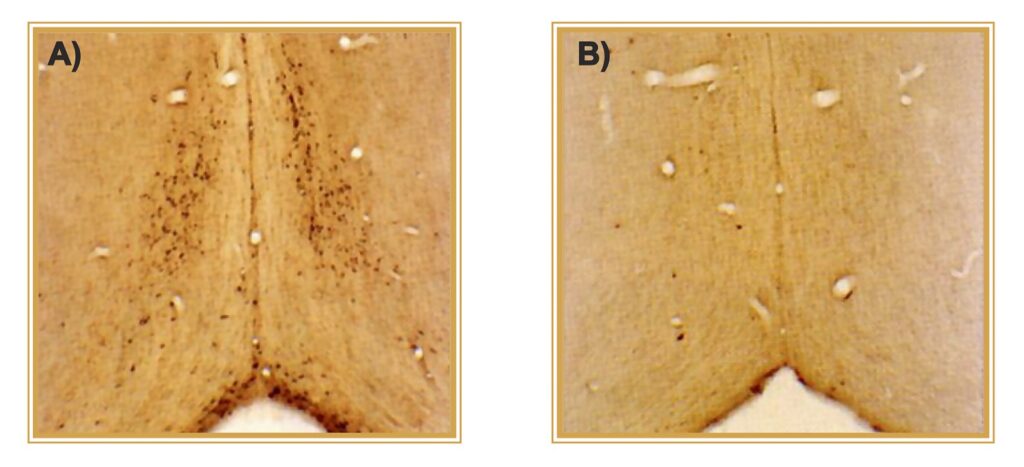
(A) CONTROL. p75NTR-positive neurons of rat cholinergic forebrain.
(B) TREATED. One icv injection of 192-IgG-SAP results in >95% elimination of target neurons.
Photo courtesy of C. Wrenn, R.G. Wiley
| Saporin Conjugate | Molecular Target of Toxin | Targeting Moiety | Animal Model or Major Research Area | Reference |
| 192-IgG-SAP (rat) | Nerve growth factor receptor | Antibody | Alzheimer’s Disease | 17 |
| Acetylated LDL-SAP | Scavenger receptor A type I/II | Protein | Alzheimer’s Disease | 25 |
| Anti-ChAT-SAP | Choline acetyltransferase | Antibody | Cholinergic System | 26 |
| Anti-DAT-SAP | Dopamine transporter | Antibody | Parkinson’s Disease | 27 |
| Anti-DBH-SAP | Dopamine-ß-hydroxylase | Antibody | Alzheimer’s Disease | 28 |
| Anti-GAT1-SAP | GABA transporter 1 | Antibody | GABA System | 29 |
| Anti-SERT-SAP | Serotonin re-uptake transporter | Antibody | Serotonin System | 30 |
| Anti-vGAT-SAP | Vesicular GABA transporter | Antibody | GABA System | 31 |
| Bombesin-SAP | Bombesin receptors (NMBR, GRPR, and BRS3) | Peptide | Itch | 32 |
| CCK-SAP | CCK receptors (CCKAR and CCKBR) | Peptide | Hunger and Appetite | 33 |
| Cholera toxin B-SAP | GM1-ganglioside | Protein | Amyotrophic Lateral Sclerosis | 34 |
| CRF-SAP | CRHR1 and CRHR2 receptors | Peptide | Neuropsychiatric Conditions | 35 |
| Dermorphin-SAP | mu opioid receptor | Peptide | Pain | 36 |
| Exenatide-4-SAP | Glucagon-like peptide-1 receptor | Peptide | Hunger and Appetite | 37 |
| FGF-SAP | FGF receptors | Protein | Cancer | 38 |
| Galanin-SAP | Galanin receptors | Peptide | GABA System | 39 |
| IB4-SAP | α-D-galactose | Lectin | Pain | 40 |
| Leptin-SAP | Leptin receptor | Peptide | Hunger and Appetite | 41 |
| Mac-1-SAP | Integrin alpha M | Antibody | Immune System | 42 |
| ME20.4-SAP (primates) | Nerve growth factor receptor | Antibody | Alzheimer’s Disease | 43 |
| Melanopsin-SAP | Melanopsin | Antibody | Circadian Rhythm | 44 |
| MOA-SAP | Blood group B antigens | Lectin | Glomerular Endothelial Injury | 45 |
| mu p75-SAP (mice) | Nerve growth factor receptor | Antibody | Alzheimer’s Disease | 46 |
| NKB-SAP | Neurokinin 3 receptor | Peptide | Reproductive System | 47 |
| NMB-SAP | Neuromedin B receptor | Peptide | Pain | 48 |
| Nppb-SAP | Atrial natriuretic factor receptors (NPRA and NPRB) | Peptide | Itch | 49 |
| NPY-SAP | NPY receptors | Peptide | Hunger and Appetite | 50 |
| Orexin B-SAP | OX1 and OX2 receptors | Peptide | Narcolepsy and Insomnia | 51 |
| OX7-SAP | Thy-1 glycoprotein | Antibody | Alzheimer’s Disease | 52 |
| Oxytocin-SAP | Oxytocin receptors | Peptide | Hunger and Appetite | 53 |
| QD-SAP | Macrophage scavenger receptor 1 and Mannose receptor | Quantum Dot | Parkinson’s Disease | 54 |
| Substance P-SAP | Neurokinin 1 receptor | Peptide | Epilepsy and Pain | 55 |
Saporin Secondary Conjugates and Streptavidin-ZAP
Immunotoxins made with Saporin have provided robust tools, especially in the fields of neuroscience, [16,21,22] and as studies in these fields continue to expand, finding a modular way of creating the immunotoxins became more desirable. Not all laboratories are created equal and having the scientific background, time, and funds needed to produce these tools were sometimes unavailable.
For the synthesis of an immunotoxin, choosing the right antibody is key; not all antibodies are suitable for use in saporin secondary conjugates. Particularly in the area of drug development, large numbers of antibodies need to be screened, so in 2000, a tool was developed for evaluating antibody efficacy for use as an immunotoxin. The idea was first suggested by Till et al., [23] but was not commercially available. A similar molecule was prepared at Advanced Targeting Systems (ATS) for commercial use. This research tool is called Mab-ZAP. [24] Mab-ZAP consists of a polyclonal goat anti-mouse IgG antibody chemically conjugated to saporin. Its mechanism of action is simple: Mab-ZAP is missed with an antibody candidate, the complex binds to a cell-surface antigen, and is internalized. Once internalized, saporin inactivates the ribosomes, protein synthesis ceases, and results in cell death. Mab-ZAP doesn’t have a way to enter a cell on its own and so only the cells expressing the specific antigen recognized by the primary antibody candidate are affected. Mab-ZAP uses a bivalent antibody, consisting of both the Fab and Fc region of IgG and is capable of recognizing whole IgG, not discriminating between the Fab or Fc region. Mab-ZAP was the first secondary conjugate in an arsenal of tools that ultimately eliminate time-consuming and expensive steps of chemically linking each antibody candidate directly to saporin. Instead, and immunotoxin is simply made by mixing Mab-ZAP with a primary antibody and added to cells in culture.
Subsequently, more saporin secondary conjugates were developed to expand the range of species detected. Initially, these tools utilized whole molecule IgG secondary antibodies that recognize both heavy and light chains of primary antibodies, followed by conjugates designed using monovalent antibodies that would continue to recognize whole IgG but lacked components that would contribute to ‘cap formation,’ a possible limitation due to the bivalent nature of an antibody where cross-linking could occur on the cell surface. This ‘cap’ could potentially induce some levels of endocytosis that would lead to cytotoxicity and produce a false positive for internalization of a primary antibody (Fig 4).
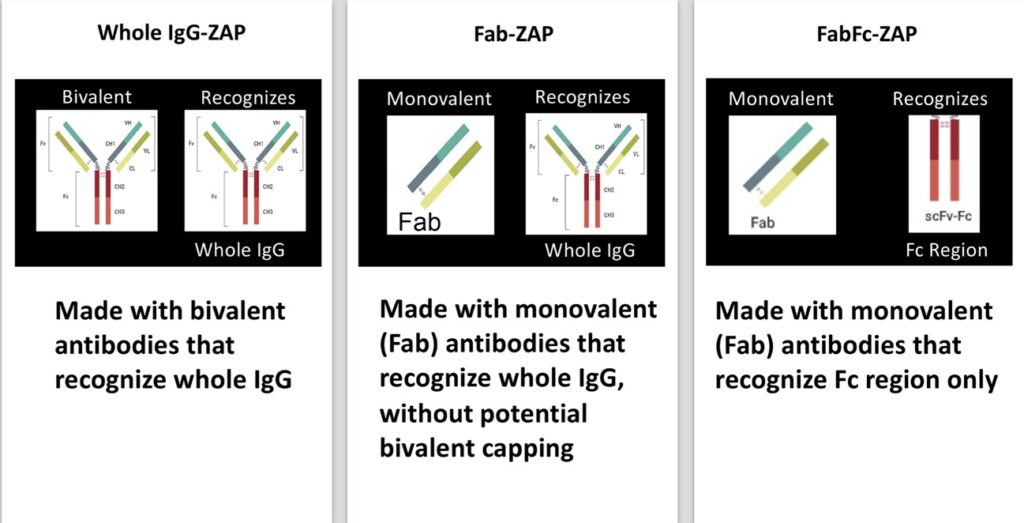
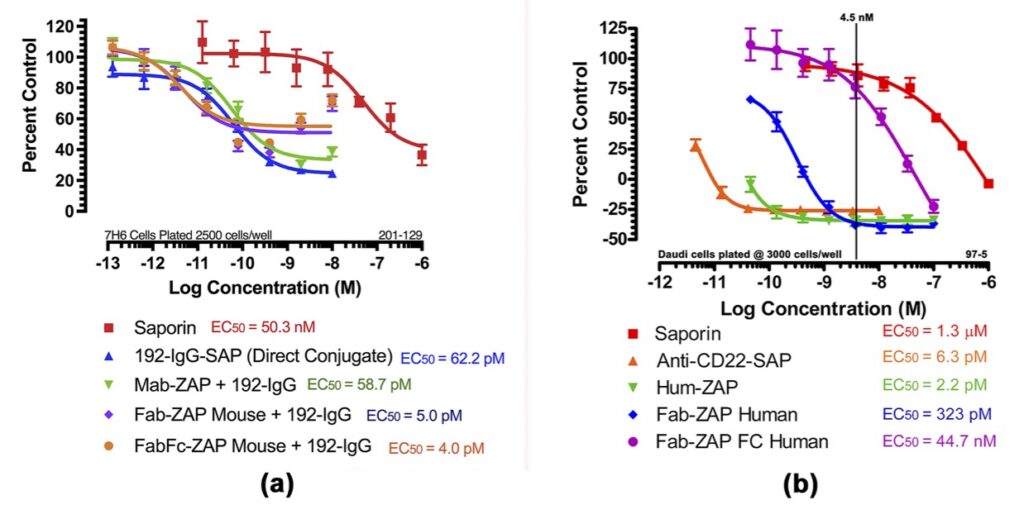
In Fig 5a, Mab-ZAP + primary antibody complex produced a similar potency to the direct-linked conjugate of saporin to the same antibody and, more interestingly, Fab-ZAP and FabFc-ZAP + primary antibody produced a cytotoxic effect >12-fold over the direct conjugate. Fig 5b also depicts an interesting phenomenon with saporin secondary conjugates. A stoichiometric effect is seen when using Fab-ZAP (blue-diamond line) and FabFc-ZAP (purple-circle line) held at constant concentration (4.5 nM) reacted with the titrated mouse monoclonal 192-IgG. It may be intuitive to think that using a higher dose of primary antibody would induce a higher amount of cell death, but as seen in Fig 5b, at the highest concentration of 192-IgG (10nM = Log -8) you can see a lessened amount of killing as compared to the antibody at a 25-fold lower concentration. The explanation for this is that at the higher concentrations of primary antibody, there is more free 192-IgG and fewer 192-IgG+Fab-ZAP complexes. The free 192-IgG out-competes the conjugates for cell surface-binding sites which in turn decreases the amount of saporin being internalized, hence less cell death.
References
History of Saporin references.