ATS Animal Models
ATS offers many different Animal Model products and full kits for various disease and behavioral research applications.
Animal Models
Learn more about our Saporin-produced Animal Models. Choose the product links below for the Animal Model of your interest. Most Animal Models are ready in 2 weeks using our Saporin conjugate system to “knock out” specific cell types to create a model of disease or behavior.
Contact us now to get started discussing the strategy that best meets your needs.
Click Animal Models below to jump to each section
Independent report of the models made with Saporin conjugates
Saporin from Saponaria officinalis as a tool for experimental research, modeling, and therapy in neuroscience.
Bolshakov AP, Stepanichev MY, Dobryakova YV, Spivak, YS, Markevich, VA (2020) Saporin from Saponaria officinalis as a tool for experimental research, modeling, and therapy in neuroscience. Toxins (Basel) 12(9):546. doi: 10.3390/toxins12090546
Summary: A review of studies where saporin-based conjugates were used to analyze cell mechanisms of sleep, general anesthesia, epilepsy, pain, and development of Parkinson’s and Alzheimer’s diseases.
Alzheimer’s Disease
Rat
Tool for eliminating cells that express p75NTR in rat; targeted via the antibody to NGFR (192-IgG), eliminated via saporin
Mouse
Tool for eliminating cells that express p75NTR in mouse; targeted via NGFR affinity-purified rabbit polyclonal antibody, eliminated via saporin
Primates
Tool for eliminating cells that express p75NTR in multiple species; targeted via NGFR monoclonal antibody (ME20.4), eliminated via saporin
Rat
Tool for eliminating cells that express dopamine beta-hydroxylase in rat; targeted via the antibody to dopamine beta-hydroxylase (DBH),
eliminated via saporin

Alzheimer’s Disease models
A review of the tools for creating animal models of Alzheimer’s Disease
“192 IgG-Saporin binds selectively and irreversibly to low-affinity nerve growth factor receptor interrupting cholinergic neuronal protein synthesis.”
“Anti-DBH-SAP allows a selective and gradual lesioning of noradrenergic neurons in the brain stem nucleus locus coeruleus, the primary site of noradrenaline production in the CNS.”
back to top
Narcolepsy and Insomnia
Rat
Tool for eliminating cells that express the orexin-2 receptor; targeted via orexin-B peptide, eliminated via saporin
The orexin A and orexin B receptors (also known as hypocretin 1 and 2) are found in the perifornical area/latero-posterior hypothalamus, and projections from this area cover much of the brain. These receptors have been implicated in various neurophysiological and neuropsychological disorders such as narcolepsy, insomnia, drug addiction, anxiety, and migraine headaches. The orexin-B-SAP conjugate consists of the rat/mouse orexin-B peptide conjugated to saporin. Orexin-B binds to the hypocretin (HCRT)-2 receptor with approximately 5-times greater affinity than to the HCRT-1 receptor.
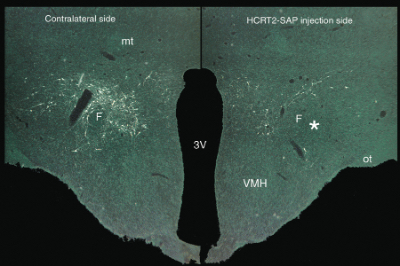
Orexin-SAP (50 ng/0.5 μl) delivered to the lateral hypothalamus kills the orexin/hypocretin receptor-positive neurons. The asterisk marks the site of injection. VMH=ventromedial hypothalamus; F=fornix; 3v=third ventricle
Narcolepsy Publications
Explore these useful articles and publications
for more information:
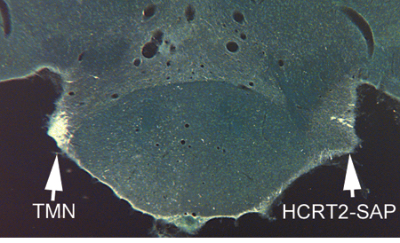
Loss of histaminergic neurons in the tuberomammillary nucleus (TMN) after unilateral injection of orexin-SAP (50 ng/0.5 μl). The TMN neurons contain the orexin/hypocretin receptor and are heavily innervated by hypocretin fibers.3 HCRT2-SAP (hypocretin 2-Saporin) = orexin-SAP
back to top
Temporal Lobe Epilepsy
Rat
Tool for eliminating cells that express substance P receptor (NK-1); targeted via Substance P, eliminated via saporin
Targeted hippocampal GABA neuron ablation by Stable Substance P-Saporin (SSP-SAP; Cat. #IT-11) initiated a subclinical state of non-convulsive status epilepticus, which produced hippocampal sclerosis and dentate granule cell-onset epilepsy, without involving convulsive status epilepticus or any lethality. This model is stable for at least one year.
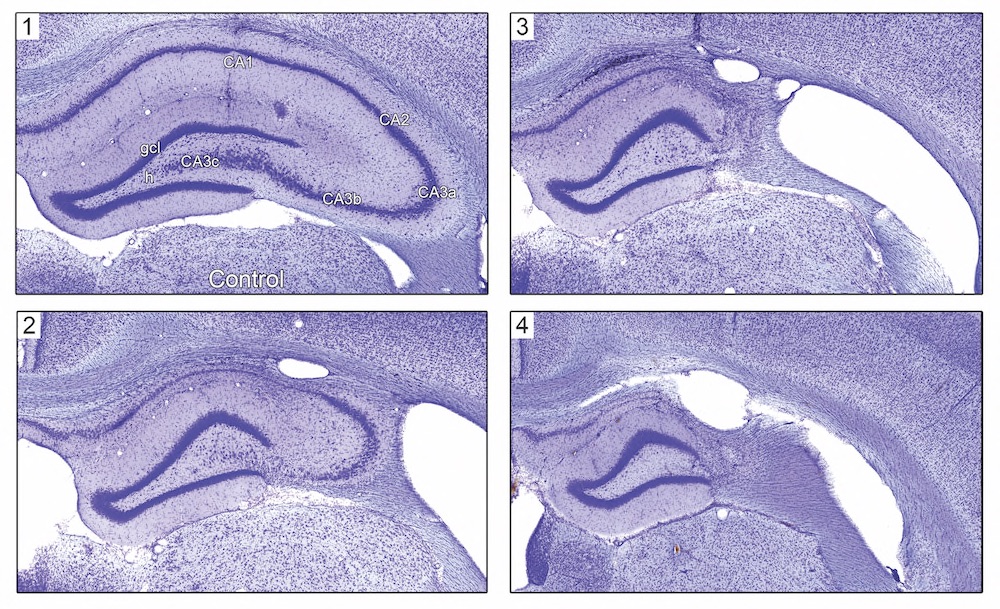
Fig. 1. Hippocampal pathology ~1 year after SSP-SAP injection. Nissl-stained hippocampi from treated rats (panels 2-4) showing variable hippocampal sclerosis pathology compared to a PBS vehicle-injected control animal (panel 1). Note the loss of neurons in the hilus (h) of the dentate gyrus, CA3, and CA1.
Temporal Lobe Epilepsy Animal Model
In this study, we determined whether selective hippocampal GABA inhibitory interneuron loss produced a chronic epileptic state and assessed cognitive function in chronically epileptic SSP-SAP-treated rats and vehicle-injected controls to identify behavioral co-morbidities associated with GABA neuron ablation. Male Sprague Dawley rats (350-450 g) were injected bilaterally with SSP-SAP (0.4 ng/10 nL) or PBS (vehicle control) into 4 sites along the longitudinal axis of each hippocampus. Receptor-mediated lesioning with SSP-SAP is highly selective because the neurotoxin Saporin enters GABA neurons via the NK-1 receptor, which all hippocampal GABA neurons constitutively and selectively express. Cognitive function was assessed in chronically epileptic SSP-SAP-treated rats and their vehicle-injected controls ~8 months post-injection, when treated rats were observed to exhibit spontaneous clinical focal motor seizures.
back to top
Parkinson’s Disease
Rat
Tool for eliminating cells that express dopamine transporter (DAT-ECD) in rat or human; targeted via a monoclonal antibody to the second extracellular loop of DAT, eliminated via saporin
One of the main ways to research Parkinson’s Disease (PD) is to use the neurotoxin 6-hydroxydopamine (6-OHDA), which selectively destroys dopaminergic and noradrenergic neurons in the brain and can induce Parkinsonism in laboratory animals. Although a valuable tool, there are issues with using this model such as not producing extra-nigral pathology or Lewy body-like inclusions. A Saporin conjugate, such as Anti-DAT-SAP or Anti-DBH-SAP can also lesion dopaminergic neurons. It’s important to note the that the lesioning becomes more specific than 6-OHDA because the lesioning only occurs in places that express the dopamine transporter or dopamine-beta hydroxylase.
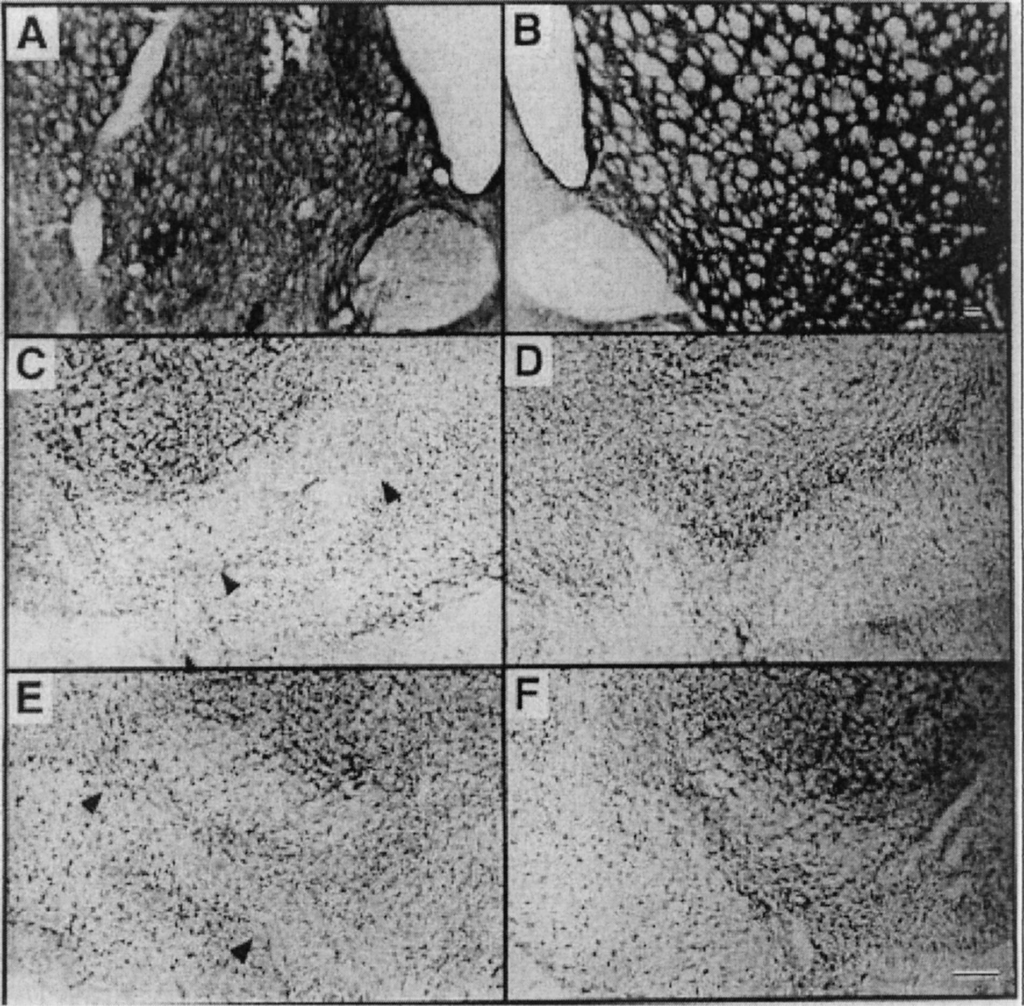
Parkinson’s Disease Models
Representative sections taken 2 weeks after intrastriatal injection of anti-DAT-saporin (0.28 µg). Panels A and B are stained for tyrosine hydroxylase. Panels C–F are Nissl stained with cresyl violet. Panels A, C, and E are ipsilateral to the striatal injection. Panels B, D and F are from the contralateral side of the same sections. Magnification bars indicate 100µm; the bar in panel B applies to panels A and B while the bar in panel F applies to panels C–F. Note loss of cells from substantia nigra, pars compacta in panels C and E (arrowheads). Also, panel A shows obvious local tissue damage around the injection site.
Wiley R.G., Harrison M.B., Levey A.I., Lappi D.A. Destruction of midbrain dopaminergic neurons by using immunotoxin to dopamine transporter. Cell. Mol. Neurobiol. 2003;23:839–850. doi: 10.1023/A:1025065306264.
back to top
Lou Gehrig’s Disease
(Amyotrophic lateral sclerosis, ALS)
Rat
Tool for eliminating cells that express the GM1 receptor; targeted via cholera toxin B-subunit, eliminated via saporin
Amyotrophic lateral sclerosis (ALS), also called Lou Gehrig’s disease, is centered around motor neuron deterioration. When these neuron’=s die, muscles can no longer function. Cholera toxin subunit B conjugated to Saporin (CTB-SAP) has a specificity towards motor neurons via GM1 ganglioside and has been used in different varieties to mimic complications in ALS.
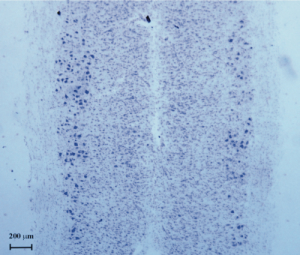
This model of spinal cord (SC) motoneuron degeneration, induced by CTB-SAP, represents a useful tool for future studies attempting to investigate neurogenesis and/or other compensatory changes within the SC, in the presence of only neurodegenerative processes, without other microenvironmental cues such as inflammation, tissue damage, disruption of SC white matter and blood circulation.
Lou Gehrig’s Disease Models
Effect of CTB-SAP on the number of surviving motoneurons in the lumbar SC, one week after lesion, as observed in cresyl violet-stained SC sections obtained from unilaterally injected animals (n=5). The same results were found one month after lesion. The original picture has been adjusted in brightness and contrast.
