Background of General Preparation and Components:
Over the last few years, riding on the coattails of the established customer base for the ZAP line of secondary conjugates, Fab-ZAP has become the most popular ATS product group in the catalog. Combining species-specific monovalent secondary antibodies with the cytotoxic ribosome-inactivating protein, Saporin, the Fab-ZAP line of products provides researchers with an ideal tool for quick diagnostic analysis of primary antibody internalization with their predicted receptors without having to worry about theoretical false-positive non-specific cell death via bivalent antibody capping.
One drawback that ATS has recently addressed within the Fab-ZAP product line is the use of these secondary conjugates with B-cells, particularly popular are those of non-Hodgkin’s lymphoma. B-cells express endogenous surface immunoglobulins (sIg), mostly in the form of monomeric IgM that play a significant role in antigen recognition and immune-response activation. The sIg is anchored to the B-cell surface in both naïve and activated B-cells by the Fc region of the molecule while the Fab portion of the sIG could be bound by external human secondary antibodies, including those used in the ATS secondary ZAP conjugates, resulting in unintended B-cell death.
ATS has now released for sale Fc specific Fab-ZAP conjugates for use with two species of primary antibodies, human and mouse. The secondary antibody used in the Fc-specific Fab-ZAP will react only with the Fc portion of the IgG heavy chain. These conjugates have been tested by ELISA and/or absorbed against Fab fragments. These new FabFc-ZAP products can be used in B-cell research with the confidence that cell death is primary antibody-mediated.
Methods of Cytotoxicity Assessment:
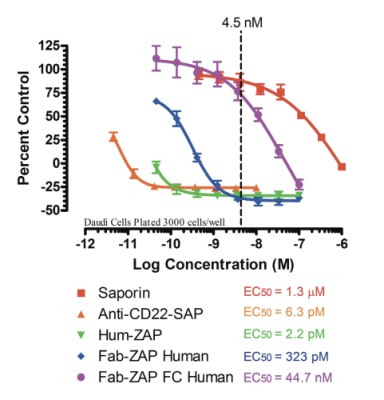
To test the new material, we first focused on the human specific versions and performed a 5-day in-vitro cytotoxicity assay to compare our already established line of secondary conjugates Hum-ZAP (Cat. #IT-22), and Fab-ZAP Human (Cat. #IT-51) vs. the newly developed FabFc-ZAP Human (Cat. #IT-65), on Daudi cells, a human B-lymphoblast. The assay would demonstrate that the specificity of this new product line can remedy issues seen with Hum-ZAP and Fab-ZAP recognizing endogenous surface immunoglobulins and unintentionally eliminating cells to provide a new tool in B-lymphocyte research. (For the complete protocol visit our website: www.atsbio.com/protocols.)
Results and Discussion:
Analysis of these data demonstrate a firm correlation with what was expected: Hum-ZAP and Fab-ZAP Human are excellent at binding human IgG, so much so that we don’t recommend their use with B-cells because they exhibit unintended binding with endogenous surface immunoglobulins.
The conjugate Hum-ZAP displayed the most B-cell cross-reactivity with an EC50 of 2.2 pM, followed by Fab-ZAP Human with an EC50 of 323 pM. The EC50 for the new Fc-specific FabFc-ZAP was calculated at 44.7 nM, showing to be less potent by a magnitude of 100-fold under the previous Fab-ZAP Human and a 20,000-fold difference under Hum-ZAP.
The recommended use of our secondary conjugates is approximately 4.5 nM. This concentration point has been depicted on the graph by a dotted-line and clearly demonstrates the difference between the newly developed FabFc-ZAP (Purple Line) vs. previous secondary conjugates and controls and their reaction when specifically studying B-lymphoblasts.
Our goal is to continue providing our customers with the tools they need to advance their science and we look forward to the future challenges. It’s these challenges that give us the chance to work with our customers to improve our products and create new ones.