Dr. Robert Sloviter, University of Arizona, contributes this issue’s article from the laboratories of ATS customers. Dr. Sloviter summarizes his research with SSP-saporin, which he and his graduate student Jennifer Martin used to examine the role of inhibitory neurons in maintaining normal network excitability.
The mammalian hippocampus is perhaps the most intensely studied brain region for a variety of reasons. Hippocampal structure and function are highly conserved among mammalian species, and its highly laminar organization greatly facilitates experimental design and interpretation. However, its greatest attractions are its involvement in the normal functions of learning and memory, and in a variety of neurological disorders including stroke, Alzheimer’s Disease, and epilepsy. One of the major issues of hippocampal research involves the structure and function of hippocampal inhibitory interneurons, and how they determine the behavior of excitatory hippocampal principal cells. We and others have sought to determine whether certain network behaviors might be the result of inhibitory neuron dysfunction or loss, but it has always been difficult to remove or disable inhibitory neurons selectively, without producing significant collateral damage.
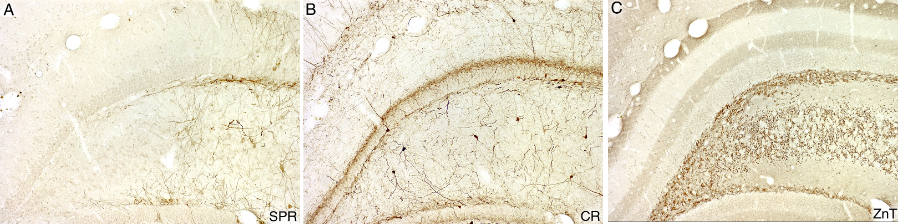
Selective loss of Substance P receptor (SPR)-immunoreactive cells after intrahippocampal injection of SSP-SAP.(A) All SPR-positive cells and dendrites have been ablated on the left side of the photograph. (B) Calretinin (CR)-immunoreactive cells and fibers in an adjacent section survive in the SPR depletion zone. (C) In another adjacent section, zinc transporter-3 (ZnT3)-positive terminals are similarly unaffected in the SPR depletion zone.
After some failed attempts to lesion specific neuronal populations, we were excited to read the paper by Pat Mantyh and his colleagues,1 in which they reported the efficacy of Substance P-saporin (SP-SAP) for removing SP receptor- positive cells in the spinal cord. Because some hippocampal inhibitory interneurons had been reported to express SP receptors (SPRs),2 we purchased SP-SAP, hoping to use this approach in the hippocampus. However, we discovered in pilot experiments that SP-SAP, when injected directly into the hippocampal parenchyma, did not diffuse sufficiently far from the injection site to destroy interneurons in an area large enough for our purposes. Fortunately, ATS had just developed a conjugate using a peptidase- resistant SP analog (SSP-SAP), which we obtained and tested while we conducted an anatomical study designed to determine exactly which hippocampal interneurons constitutively express SPRs, and should therefore be vulnerable to SSP-SAP. That study demonstrated that most inhibitory neurons of all hippocampal subregions expressed SPRs, and that no excitatory principal cells or glia were SPR- positive.3
We found that 10 nl of a solution containing less than 1 ng of SSP-SAP was capable of selectively eliminating all SPR-positive neurons within a 2-mm diameter sphere of tissue. The survival of SPR-negative elements within the SPR depletion zone was remarkable and included excitatory neurons, glia, myelinated fibers, and a number of afferent fiber systems originating outside the hippocampus. Selective loss of SPR- positive inhibitory interneurons was associated with a highly focal disinhibition and hyperexcitability4 that was clearly not caused by a global neurological insult that invariably causes a myriad of non-specific pathologies. Our results indicate that epileptiform behavior is intrinsic to the hippocampal network and does not require the principal cell loss or synaptic reorganization that other models of network hyperexcitability exhibit as a result of less specific neurological injuries. At the least, our results clearly indicate that SSP-SAP will be an extremely useful tool for a wide variety of studies in the hippocampus and other SPR-positive brain regions.
References
1. Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA (1997) Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278:275-279.
2. Acsády L, Katona I, Gulyas AI, Shigemoto R, Freund TF (1997) Immunostaining for substance P receptor labels GABAergic cells with distinct termination patterns in the hippocampus. J Comp Neurol 378:320-336.
3. Sloviter RS, Ali-Akbarian L, Horvath KD, Menkens KA (2001) Substance P receptor expression by inhibitory interneurons of the rat hippocampus: enhanced detection using improved immunocytochemical methods for the preservation and colocalization of GABA and other neuronal markers. J Comp Neurol 430:283-305, 2001.
4. Martin JL, Sloviter RS (2001) Focal inhibitory interneuron loss and principal cell hyperexcitability in the rat hippocampus after microinjection of a neurotoxic conjugate of saporin and a peptidase-resistant analog of Substance P. J Comp Neurol 436:127-152.
