Over the years, ATS has frequently been asked about Saporin’s safety for use in the lab as well as when used clinically. Residual awareness of alternate Ribosome-Inactivating Proteins (RIPs) and ‘toxins’ such as Ricin have caused some researchers new to the use of RIPs to question the belief that Saporin is safe. Unlike Type 2 RIPs (such as Ricin), Type I RIPs, like Saporin have no binding chain and consequently no means of entering the physiological space necessary for the protein to act as a toxin. The following is a review of safety in handling and potential toxicity within the human body for systemic events not related to normal research applications of Saporin conjugates, including Substance P-Saporin (SP-SAP), which is a therapeutic under development for the treatment of chronic pain.
The acute LD50 for saporin in mice (25 g) is 6.8 mg/kg;[1] that would translate in humans (75 kg) to 510 mg! A concentration of about 100 nM is the threshold to see even a vague hint of saporin toxicity. In human blood, that would correspond to 24 mg injected systemically into a person. The fermentation process to produce recombinant saporin has a titer of 2 mg/L meaning that the production broth itself contains no more than 67 nM concentration of saporin. Furthermore, the final protein concentrations from production batches of recombinant Saporin used in our drug are 4 mg/ml, meaning 6 mL of final material would need to accidentally end up in a human before the ‘hint of toxicity’ threshold would potentially be met.
The toxicology studies of SP-SAP contained within ATS’s IND prior to the current human Phase I clinical trial evaluated effects related to the intended method of administration, intrathecal local injection. SP-SAP is not expected to ever be a self-administered therapy, so the effects of gross off-target events, such as accidental auto-injection, swallowing, spillage, or immersion were not considered.
The table below[2] highlights antibody-saporin conjugates approved by the FDA for Phase I/II clinical trials in humans. The therapeutics listed below were administered intravenously and imply what the FDA accepted as non-toxic levels of saporin-based conjugates in these studies.
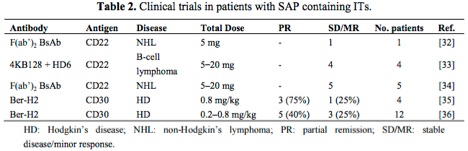
Looking more closely at the study by French et al.,[3] several milligrams of antibody conjugate were repeatedly injected into human patients under a FDA regulated clinical trial and peak serum levels tested, demonstrating rapid clearing of saporin from the system.
As a company that specializes in Saporin, our two-plus decades of experience working with the protein in research, preclinical, and clinical environments has taught us that with minimal standard laboratory precautions users are not at any real risk of toxic effects. Even our CSO, after 30+ years of working with Saporin exhibits undetectable levels of Saporin antibodies in his blood!
References:
- Thorpe PE et al. An immunotoxin composed of monoclonal anti-thy 1.1 antibody and a ribosome-inactivating protein from Saponaria officinalis: potent antitumor effects in vitro and in vivo. J Natl Cancer Inst 75:151-159, 1985.
- Polito L et al. Immunotoxins and other conjugates containing saporin-s6 for cancer therapy. Toxins (Basel) 3(6):697-720, 2011.
- French RR et al. Response of B-cell lymphoma to a combination of bispecific antibodies and saporin. Leuk Res 20(7):607-17, 1996.
