Phrenic long-term facilitation following intrapleural CTB-SAP-induced respiratory motor neuron death. Nichols NL, Craig TA, & Tanner MA. Respir Physiol Neurobiol. 2018 Oct;256:43-49.
doi: 10.1016/j.resp.2017.08.003. Epub 2017 Aug 16.
There are currently no approved treatment options to significantly preserve/restore breathing capacity in patients with Amyotrophic lateral sclerosis (ALS). Intrapleural CTB-SAP (Cat. #IT-14) mimics aspects of ALS. This model of respiratory motor neuron death may provide the ability to harness mechanisms of respiratory motor plasticity to increase and preserve the function of surviving motor neurons.
Also see: Targeted Ablation of Sympathetic Neurons Reduces Ventricular Arrhythmias and Autonomic Dysreflexia. Heidi L. Lujan and Stephen E. DiCarlo. Targeting Trends (2015).
CTB-SAP retrogradely transported from the peripheral ganglia is effective at ablating specific sympathetic neurons and reducing the susceptibility to ventricular arrhythmias and AD. Additional studies are required to further characterize the physiological responses to this procedure as well as determine if this new approach is safe and efficacious for the treatment of conditions associated with excess sympathetic activity.
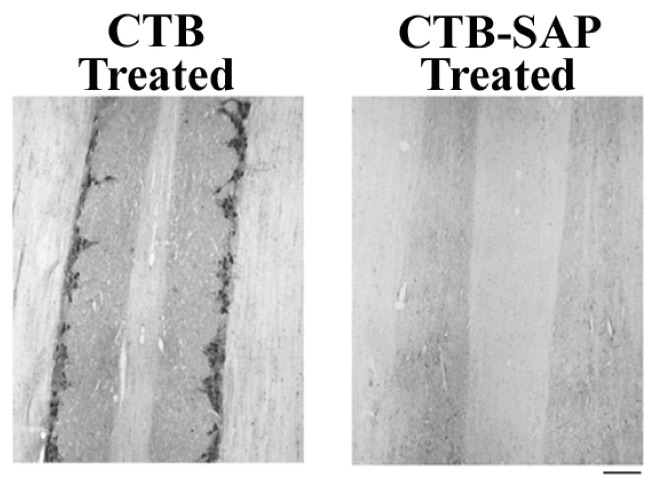
*P < 0.05, CTB vs. CTB-SAP.
CTB-SAP causes ablation of any cell type expressing GM1, including preganglionic neurons, motoneurons, and astrocytes. Intrathecal injection of CTB-SAP results in elimination of oligodendrocytes and astrocytes and the subsequent demyelination of the spinal cord. CTB-SAP allows the study of a number of demyelinating conditions.
CTB-SAP eliminates cells that express the receptor to Galactosyl-N-Acetylgalactosaminyl.
