SSP-SAP
[Stable analog of Substance P conjugated to Saporin]
(Cat. #IT-11)
Objective: To determine the the mechanisms mediating the induction of opioid-induced hyperalgesia and the prolongation of prostaglandinE2-induced hyperalgesia in type II hyperalgesic priming.
Summary: The authors show that understanding the mechanisms responsible for the induction of type II hyperalgesic priming, a form of neuroplasticity in the peripheral terminal of the primary afferent nociceptor, may provide useful information for the design of drugs with improved therapeutic profiles to treat neuroplasticity induced by chronic use of opioids.
Methods: SSP-SAP was prepared in saline (5 ng/mL), and 20 mL was injected intrathecally into rats, 14 days before nociceptive tests.
Araldi D, Ferrari LF, & Levine JD. Role of GPCR(Mu-Opioid)-Receptor Tyrosine Kinase (Epidermal Growth Factor) Crosstalk in Opioid-Induced Hyperalgesic Priming (Type Ii). (2018). Pain, in press (see Figure 3), PMID: 29447132 DOI: 10.1097/j.pain.
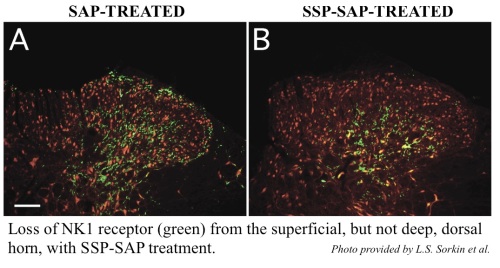
SSP-SAP (Cat. #KIT-11) specifically targets cells that express substance P receptor (NK-1).
SSP-SAP is a conjugation of saporin and SSP, the Sar9, Met(O2)11 analog of Substance P. These two amino acid replacements are at two sites of digestion by tissue proteases (there is a third that is still in the analog, so it does eventually get degraded). Since the targeting agent is, of course, necessary for the cytotoxic activity, SSP-SAP will diffuse farther, and thus hit more target cells. This specific analog of SP-saporin resists peptidase digestion, allowing a greater diffusion from the injection site before its metabolism. SSP-SAP eliminates cells expressing the Substance P (NK-1) receptor. Behaviors associated with pain perception are greatly affected by the injection of SSP-SAP into the spinal cord of rats. It is not suitable for retrograde transport.
This kit includes SSP-SAP (Cat. #IT-11) plus Blank-SAP (Cat. #IT-21).
