The Combinational Use of CRISPR/Cas9 and Targeted Toxin Technology Enables Efficient Isolation of Bi-Allelic Knockout Non-Human Mammalian Clones. Watanabe S, Sakurai T, Nakamura S, Miyoshi K, & Sato M. (2018). Int J Mol Sci, 19 (4).
Objective: Most genome editing systems employ transient treatment with selective drugs such as puromycin to obtain the desired genome-edited cells, which often allows some untransfected cells to survive and decreases the efficiency of generating genome-edited cells. The authors developed a novel targeted toxin-based drug-free selection system for the enrichment of genome-edited cells.
Summary: Results indicate that a combination of the CRISPR/Cas9 system and targeted toxin technology using IB4-SAP allows efficient enrichment of genome-edited clones, particularly bi-allelic KO clones.
Dose: Cells were trypsinized 3 days after transfection and approximately 80% were incubated for 30 min at 37°C in a solution (25 mcL) containing 0.5–1.0 mcg IB4-SAP (Cat. #IT-10).
IB4-SAP (Cat. #IT-10)
A tool for eliminating cells that express α-D-galactopyranoside residues in cells; targeted via recombinant Isolectin B4 (IB4), eliminated via saporin.
Isolectin B4 (IB4) is one of a family of five alpha-D-galactose-binding lectins from Griffonia (Bandeiraea) simplicifolia. Recombinant IB4* was expressed in E. coli and purified using affinity chromatography. In one important application rIB4-SAP specifically eliminates the IB4-positive c-fiber nociceptor neurons, while sparing the peptidergic neurons. Upon binding the alpha-D-galactopyranoside residues expressed on the cell surface, rIB4-SAP becomes internalized and saporin inhibits protein synthesis, resulting in elimination of the neurons.
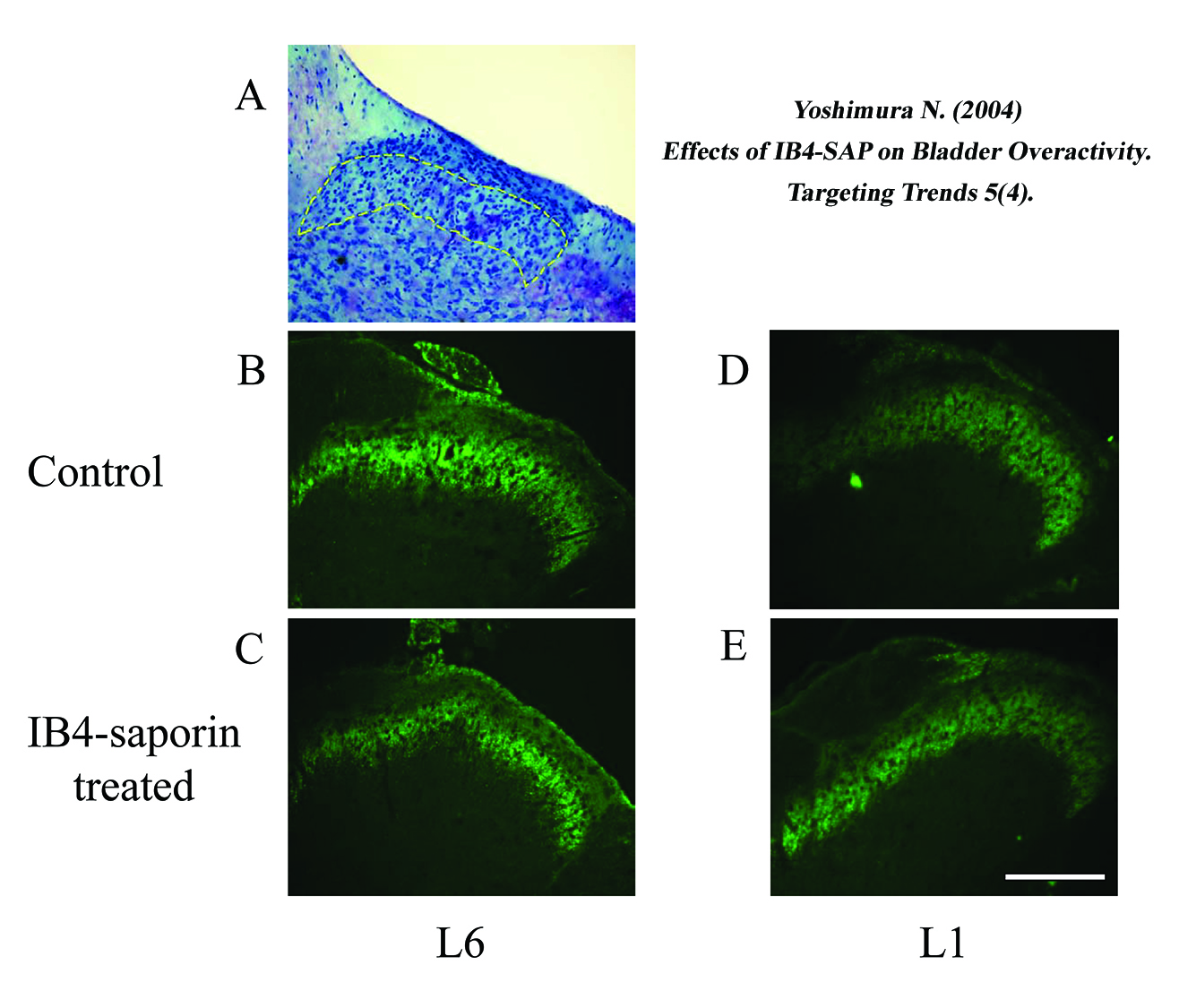
The lamina II area identified by Nissl’s staining is indicated by dashed lines in A. Note that the staining density of IB4-binding afferent nerve terminals in the lamina II of the L6 spinal cord was depleted after the IB4-SAP treatment. Scale bar, 200 μm.
