Targeted hippocampal GABA neuron ablation produces hippocampal sclerosis, epilepsy, and dissociable effects on the Morris Water Maze and Object-Place Paired Association tasks
by Argyle V. Bumanglag1, Leah M. Truckenbrod1, Eugene Chun2, Abbi R. Hernandez1, Quinten P. Federico1, Andrew P. Maurer1, Robert S. Sloviter3,4, Sara N. Burke1
1Neuroscience, McKnight Brain Institute, University of Florida, Gainesville, FL, 2Graduate Education Biomedical Sciences Program, Morehouse SOM, Atlanta, G, 3Neurobiology, Morehouse SOMe, Atlanta, GA, 4Pharmacology and Toxicology, Morehouse SOM, Atlanta, GA.
Temporal lobe epilepsy with hippocampal sclerosis is a common neurological disorder characterized by seizures that arise from the hippocampus and related structures, often as a consequence of brain injury.1 Perforant path stimulation-induced hippocampal injury in rats is associated with immediate dentate granule cell hyperexcitability and a clinically subtle, focal epileptic brain state without significant delay.2 Thus, an immediate disruption in inhibition from injury-induced neuron loss or GABA neuron dysfunction may be a sufficient cause of temporal lobe epilepsy with hippocampal sclerosis.3-4 We recently tested this hypothesis by determining whether the selective ablation of hippocampal GABA neurons along a longitudinal expanse of the hippocampus, in the absence of any other induced brain injury, is sufficient to initiate hippocampal epileptogenesis.5 The results of this experiment suggested that targeted hippocampal GABA neuron ablation by Stable Substance P-Saporin (SSP-SAP; Cat. #IT-11) initiated a subclinical state of non-convulsive status epilepticus, which produced hippocampal sclerosis and dentate granule cell-onset epilepsy, without involving convulsive status epilepticus or any lethality.5 If correct, GABA neuron loss or GABAergic dysfunction alone may be a primary epileptogenic mechanism.2-5
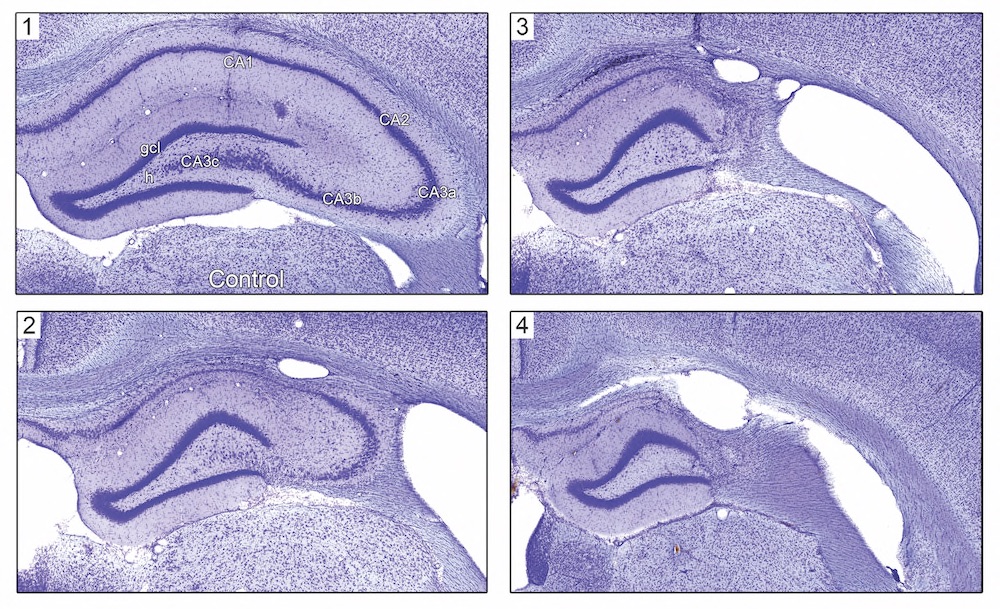
In this study, we determined whether selective hippocampal GABA inhibitory interneuron loss produced a chronic epileptic state, and assessed cognitive function in chronically epileptic SSP-SAP-treated rats and vehicle-injected controls to identify behavioral co-morbidities associated with GABA neuron ablation. Male Sprague Dawley rats (350-450 g) were injected bilaterally with SSP-SAP (0.4 ng/10 nL) or PBS (vehicle control) into 4 sites along the longitudinal axis of each hippocampus. Receptor-mediated lesioning with SSP-SAP is highly selective because the neurotoxin Saporin enters GABA neurons via the NK-1 receptor, which all hippocampal GABA neurons constitutively and selectively express.6 Cognitive function was assessed in chronically epileptic SSP-SAP-treated rats and their vehicle-injected controls ~8 months post-injection, when treated rats were observed to exhibit spontaneous clinical focal motor seizures.
Spatial learning and memory were initially assessed with the Morris Water Maze.7 No significant differences were detected in Morris Water Maze performance between SSP-SAP-treated rats and the control animals. Controls and epileptic rats with dorsal hippocampal sclerosis were able to learn the location of the hidden platform in the Morris Water Maze task, suggesting that entorhinal regions and associated areas involved in spatial navigation may have retained their functional integrity (Fig. 2).
The same animals were subsequently tested with the object-place paired association (OPPA) task, which requires animals to integrate spatial location memory with a correct object choice, and is a more sensitive measure of dysfunction within hippocampal-cortical circuits than the Morris Water Maze.8-10 SSP-SAP-treated rats exhibited significantly impaired performance on the OPPA task, and made significantly more errors before reaching criterion compared to control animals (Fig. 3A). Thus, cognitive functions that require functional connectivity between the hippocampus and cortical areas may be selectively affected by hippocampal GABA neuron ablation. No significant differences were detected on a control simple object discrimination task (Fig. 3C), suggesting that observed impairments on the OPPA task were not simply due to differences in motivation, procedural or sensorimotor deficits.
Histological analysis was performed ~12 months post-injection to determine the extent of hippocampal sclerosis in chronically epileptic rats. Targeted hippocampal GABA neuron ablation via SSP-SAP consistently caused hippocampal sclerosis (Fig. 1). Varying extents of neuron loss were observed in different rostrocaudal locations along the same hippocampus. Rats corresponding to panels 2-4 in Fig. 3 were observed to exhibit a minimum of two clinically-obvious seizures that were detected by intermittent observation. Clinically-obvious seizures were observed during feeding and cage changes, or on behavioral task trials, indicating that animals continue to be epileptic for more than a year after SSP-SAP microinjection.
These data suggest that, similar to humans with mesial temporal lobe epilepsy, hippocampal sclerosis and epilepsy in this model do not result in global cognitive decline. Rather, cognitive functions that require functional connectivity between the hippocampus and cortical areas are selectively affected. Future studies will examine the effects of selective GABA neuron ablation on hippocampal network dynamics across the lifespan, and identify cognitive co-morbidities associated with temporal lobe epilepsy and aging. The relationship between different extents of hippocampal neuron loss and performance on cognitive tasks also remains to be determined.
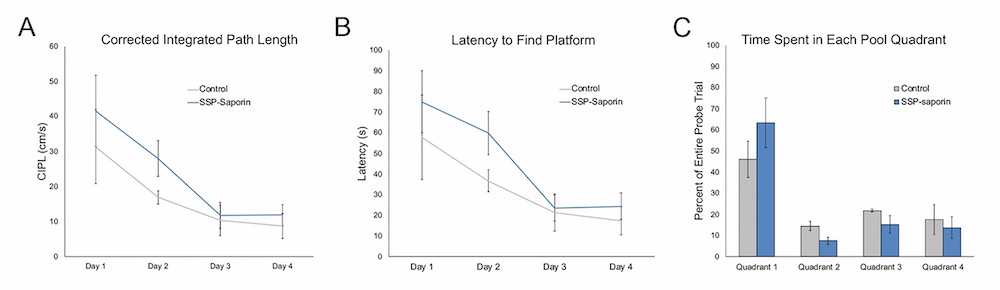
Fig. 2. Morris Water Maze performance in chronically-epileptic rats ~8 months after SSP-SAP injection. A) Average corrected integrated path length of each group across days of water maze testing. No significant differences were observed between experimental and control animals. B) Latency to find hidden platform within the water maze pool over days of water maze testing. No significant differences were found between the SSP-SAP treated rats and their control counterparts. C) Percent time spent in each pool quadrant during the probe trial. Both control and SSP-SAP treated rats spent more time in the quadrant that previously contained the hidden platform. There were no significant differences between treatment groups.
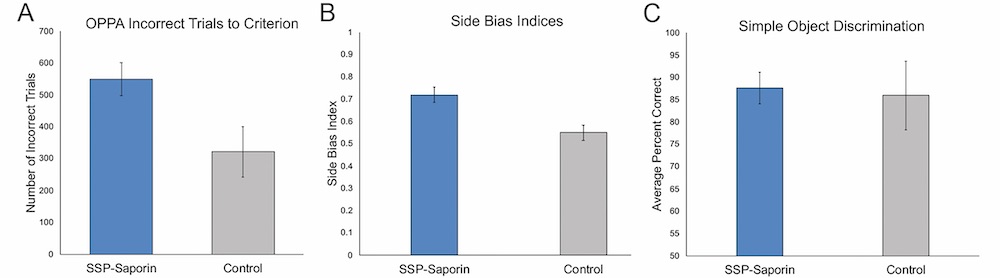
Fig. 3. Object-Place Paired Association task performance in SSP-SAP treated animals compared to control. A) Number of incorrect trials to reach criterion performance on the OPPA task. Treated rats performed greater numbers of incorrect trials (p=0.04) to reach criterion performance on the OPPA task. B) Average side bias index on the OPPA task. A value of zero indicates no bias towards either the left or right possible choices. SSP-SAP-treated rats had a trend towards greater bias to select a well on one side compared to control animals (p=0.07), indicating these rats persevered to one well side more frequently than their control counterparts. C) Average percent of correct object choices on a control simple object discrimination task. Controls and SSP-SAP treated rats could acquire a simple pair-wise object discrimination and showed no significant differences in performance (p=0.83).
References
- Engel J Jr (1996) Epilepsy Res 26: 141-150.
- Bumanglag AV, Sloviter RS (2018) Epilepsia 59: 2019-2034.
- Sloviter RS, Bumanglag AV (2013) Neuropharmacology 69:3-15.
- Sloviter RS (2017) Curr Opin Pharmacol. pii: S1471-4892(17):30037-1.
- Chun E, Bumanglag AV, Burke SN, Sloviter RS (2019) Epilepsia 60(5):e52-e57.
- Sloviter RS, Ali-Akbarian L, Horvath KD, Menkens KA (2001) J Comp Neurol 430: 283-305.
- Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Nature 297:681-683.
- Jo YS, Lee I (2010) J Neurosci 30:9850-9858
- Hernandez AR, Maurer AP, Reasor JE, Turner SM, Barthle SE, Johnson SA, Burke SN (2015) Behav Neurosci 129:599-610.
- Hernandez AR, Reasor JE, Truckenbrod LM, Lubke KN, Johnson SA, Bizon JL, Maurer AP, Burke SN (2017) Neurobiol Learn Mem 137:36-47.
