By Rahul Palchaudhuri, Ph.D., Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, Massachusetts, USA
Hematopoietic stem cell transplantation (HSCT) has been clinically used for 58 years and offers life-saving therapies for a variety of malignant and non-malignant blood disorders. Currently 50,000 transplants are performed globally per year with 90% of these for the treatment of malignancies.
Prior to receiving a transplant, a patient must be “conditioned” which serves to destroy resident hematopoietic stem cells in the marrow, in order to create niche vacancies for successful donor stem cell engraftment. Unfortunately, current conditioning strategies are non-targeted and genotoxic as they use DNA-damaging whole body irradiation and chemotherapy. As expected, these crude methods induce severe short-term and long-term conditioning-related toxicities that ultimately limit the application of hematopoietic stem cell transplantation, particularly in non-malignant conditions (e.g. sickle cell anemia, thalassemia, immunodeficiencies and autoimmune conditions).
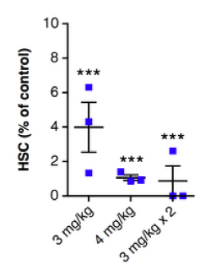
While antibodies are potentially an appealing alternative to current conditioning methods, previous antibody-based strategies relying on naked antibodies have been met with limited success in immunocompetent animals. We therefore explored antibody-based immunotoxins created using the ribosome-inactivating protein, saporin, as a means of depleting hematopoietic stem cells in immunocompetent mice. By combining various biotinylated monoclonal antibodies with streptavidin attached to saporin (Streptavidin-ZAP, Cat. #IT-27), we created immunotoxins and screened their ability to achieve stem cell depletion in vivo. From our screen, we identified CD45-SAP as a potent stem cell-depleting agent capable of depleting >98% of hematopoietic stem cells following a single-dose administration. Using CD45-SAP we demonstrated successful donor stem cell engraftment with long-term donor chimerism levels greater than 90%.
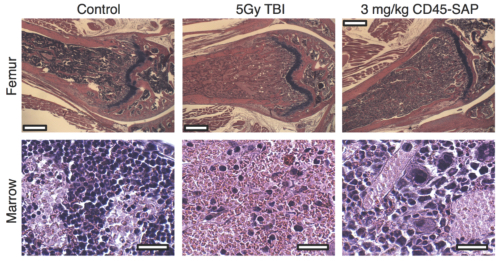
As only hematopoietic cells express the CD45 receptor, CD45-SAP offered significant advantages with regard to toxicity compared to conventional whole body irradiation. Notably, CD45-SAP enabled quicker recovery of bone marrow cellularity, avoided damage to marrow blood vessels and other non-target marrow cells, and preserved the thymic function. Combined together, these features resulted in notably quicker recovery of B- and T-cells following CD45-SAP versus irradiation. In addition, CD45-SAP avoided neutropenia, preserving innate immunity and the ability to resist fungal infection.
To demonstrate correction of a clinically relevant disease, we employed CD45-SAP in a mouse model of sickle cell anemia and demonstrated our method achieved >90% donor cell chimerism, all mice in three groups (18/18), resulting in complete disease correction (red blood cell counts, hemoglobin levels, hematocrit levels and reticulocyte frequencies were returned to normal). Fig. 2 show hematopoietic stem cell (HSC) depletion. If these pre-clinical results can be successfully translated to the clinic, it would greatly reduce conditioning-related toxicities and expand the use of hematopoietic stem cell transplantation.
Reference
- Palchaudhuri R, Saez B, Hoggatt J, Schajnovitz A, Sykes DB, Tate TA, Czechowicz A, Kfoury Y, Ruchika F, Rossi DJ, Verdine GL, Mansour MK, Scadden DT. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol. 2016 Jun 6. doi: 10.1038/nbt.3584. [Epub ahead of print] PMID: 27272386.
