by Carl Noe, M.D., Anesthesiology & Pain Management, UT Southwestern Medical Center, Dallas, TX; and Eugene McDermott Center for Pain Management, Dallas, Texas
Dr. Noe is the Principal Investigator in the ongoing trial of SP-SAP
Intrathecal SP-SAP (Substance P attached to the ribosome-inactivating protein, saporin) has been studied in a Phase 1 clinical trial of patients with cancer pain at doses of 1, 2, 4, 8, 16 and 32 mcg. The first patient was treated April 10, 2014. Doses of 64 mcg and 90 mcg remain in Phase 1a of the clinical protocol. Phase 1b will treat multiple patients at the most effective dose. To date, no toxicity has been observed and the study is ongoing to evaluate response, safety, and tolerability. (For information about the trial, please visit: http://clinicaltrials.gov/show/NCT02036281).
SP-SAP is administered via an intrathecal catheter placed in the lumbar spine with the use of fluoroscopy and radiopaque contrast injection to ensure accurate delivery of the active drug. So far, the catheter placement has been at L5 vertebral level. The same location was used in the veterinary trial conducted by Dr. Dottie Brown in dogs with osteosarcoma. [1]
The lack of side effects or toxicity have led the clinical trial team to consider possible changes to the protocol that would enhance the effectiveness of SP-SAP in treating chronic pain. A primary endpoint of the trial is: response as defined by a 20% reduction in chronic pain or opioid dose within 4 weeks of treatment. One of the patients has clearly met this endpoint with reduction of pain medication by >20% during a 4-week period, following SP-SAP treatment.
Discussions are ongoing regarding several relevant issues that may affect efficiency of drug delivery and efficacy. 1) Modification of catheter placement may produce more selective responses and reduce required doses.
2) Targeting specific spinal segments using sclerotomes (Fig. 1) may be useful in delivering SP-SAP to a spinal cord location related to the pain origination. Cancer pain may be localized primarily to a bone; in these cases, using a sclerotomal map may help guide therapy to a specific nerve root and spinal cord location.
3) Targeting specific myotomes (Fig. 2). Patients with sarcomas may have pain in a soft tissue that can be localized to a myotome and the nerve root(s) that innervate the area. Using information from the patient, physical examination, imaging and myotomal charts may help target treatment. For example, suppose a patient has a sarcoma arising from myotome-derived skeletal muscle that is predominantly innervated by the left L2 nerve root. Theoretically, a catheter could be placed posterior to where the left L2 nerve root enters the cord so that the injected SP-SAP would be close to the dorsal horn (which is the target).
4) Consideration of the baricity of SP-SAP may also be useful to more efficiently direct the placement of the drug in the spinal fluid.
Along with discussions to improve drug delivery and efficacy are considerations of the patient population being treated now (end-stage cancer patients unresponsive to opioid treatment) and future populations that could benefit from treatment with SP-SAP. In the current trial, several patients had previous spine surgery that complicated the catheter placement for intrathecal treatment. Also, patients have had heterogeneous and progressive disease and worsening pain during the study, complicating the interpretation of responses. For example, several patients reported a reduction in opioid requirements and transient pain relief. In a population where pain continues to spread along with the cancer, it is difficult to determine if the transience is due to the dose level of SP-SAP (too little) or the establishment of ‘new’ pain.
End-stage cancer patients are a needy population that desperately need relief from chronic pain. The early signs of efficacy for SP-SAP are encouraging and the next doses (64 mcg and 90 mcg) may bring the long-term pain relief needed.
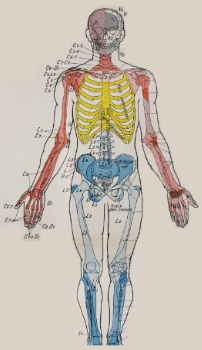
Fig. 1 SCLEROTOME TARGETING [2]
|
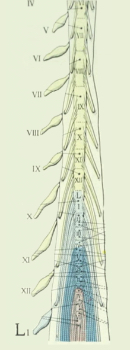
Fig. 2 MYOTOME TARGETING [2]
|
References: (back to top)
- Brown DC, Agnello K. (2013) Intrathecal substance p-saporin in the dog: efficacy in bone cancer pain. Anesthesiology 119(5):1178-1185.
- Sémiologie du système nerveux by Dejerine, J. (Joseph Jules), 1849-1917, Published 1901.
