by Aaron Kucinski (Collaborators at University Michigan, Ann Arbor: K. Phillips, R. Albin, M. Sarter)
In addition to the primary disease-defining symptoms that result from extensive loss of nigrostriatal dopaminergic neurons, approximately half of patients with Parkinson’s Disease (PD) suffer from postural instability, impairments in gait control and a propensity for falls. These symptoms have been associated with losses of cholinergic neurons situated in the basal forebrain (BF) and in the brainstem pedunculopontine nucleus (PPN). We recently developed a test system (Michigan Complex Motor Control Task, MCMCT) for the assessment of fall propensity in rats. Our initial research focusing on the modeling of falls found that cholinergic lesions of the BF in combination with striatal dopamine (DA) lesions (dual lesions, ‘DL’) generated rats with a high rate of falls that correlated with attentional impairments on an attention task¹. Given that PPN cholinergic projections have been associated with fall status in PD, we further sought to determine the contribution of PPN cholinergic loss to gait control and falls in rats with cholinergic BF and/or striatal dopamine system losses.
The MCMCT was designed to tax the ability to rapidly correct movement errors when traversing complex rotating surfaces (square rods). Rats were trained to traverse stationary and rotating rods, placed horizontally or at inclines. Traversing rotating rods and avoiding falls required persistent control of gait, limb coordination and carefully timed and placed steps. Following training, rats received cholinergic and/or striatal dopaminergic lesions or sham lesions. Cholinergic lesions were produced by bilateral infusions of 192 IgG-SAP (Cat. #IT-01) or Anti-ChAT-SAP (Cat. #IT-42) into the BF or PPN, respectively. Caudate dopaminergic deafferentation was achieved by bilateral infusions of 6-hydroxydopamine (6-OHDA) into the caudate nucleus. Following surgeries, rats were tested on a 14 day MCMCT test battery with increasingly complex traversal conditions (see Fig. 1).
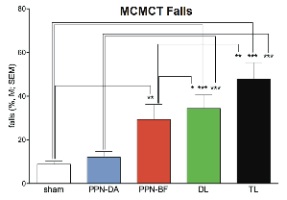
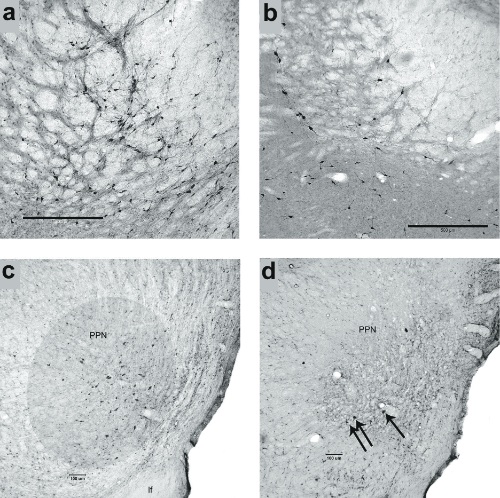
The results indicated that rats with losses of PPN and BF cholinergic neurons and striatal dopaminergic inputs fell frequently from the rods (Fig. 1), and that these falls were associated with relatively slow traversal speed and high rate of slips. The performance of rats with losses in all three regions (PPN, BF, and DA; triple lesions, ‘TL’) was not more severely impaired than following combined BF cholinergic and striatal DA lesions (DL), however, some abnormal gait characteristics were observed such as ballistic recovery movements and slip-triggered switches to symmetrical locomotion (‘galloping’). Furthermore, rats with only cholinergic cell losses (PPN and BF) fell more than shams on more complex rod traversal conditions (rod rotating in alternating directions) (Fig.1). Histological analysis showed that infusions of 192-IgG saporin into the BF removed cholinergic neurons primarily from the nucleus basalis of Meynert and the more ventral substantia innominata (Fig. 2 a,b) and anti-ChAT-SAP infusions into the PPN resulted in the almost complete loss of cholinergic neurons in this region (Fig. 2 c,d). In total, these results support a role of cholinergic systems in falls and gait control in PD and further support the hypothesis that BF cholinergic-striatal disruption of attentional-motor interactions, proposed to reflect impaired attentional control of posture, gait and movement, is a primary source of falls.
- Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M. Modeling fall propensity in Parkinson’s disease: Deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation. J Neurosci 33:16522-39, 2013.
