Contributed by Liu Y, Weick JP, Liu H, Krencik R, Zhang X, Ma L, Zhou GM, Ayala M, Zhang SC.
Waisman Center, School of Medicine and Public Health, University of Wisconsin, Madison, Wisconsin, USA. and Human Anatomy and Histology, Fudan University Shanghai Medical School, Shanghai, China.
Basal forebrain neurons including cholinergic neurons and GABA interneurons originate from the medial ganglionic eminence (MGE),[1] and play key roles in learning and memory. Dysfunction of MGE progenies results in learning and memory deficits, and may associate with many diseases including Alzheimer’s disease, Down syndrome, and dementia, none of which has an effective cure at present.[2-5] Directed differentiation of basal forebrain neurons from human pluripotent stem cells (hPSC), including embryonic stem cells (hESC)[6] and induced pluripotent stem cells (hiPSC),[7] might become a potential treatment for these learning and memory deficit diseases.
hPSCs can be maintained and proliferated long-term in a cell culture system. Under certain conditions, hPSCs can be differentiated into many types of cells of the human body, including neurons.[8-10] The process of differentiation to a certain type of neuron mimics neural development in vitro.[8] Previously, we generated various neurons from hPSCs, including cortical glutamatergic neurons,[11] midbrain dopaminergic neurons,[12,13] spinal cord motor neurons,[11,14] and striatal GABA neurons[15] by following each developmental principle.
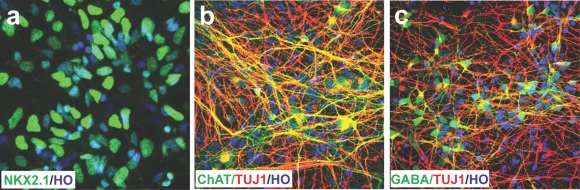
MGE cells located in the ventral forebrain express transcriptional factors FOXG1 and NKX2.1.16 Sonic hedgehog (SHH) is secreted from notochord and forms a concentration gradient from ventral to dorsal.[17,18] Therefore, a high concentration of SHH is required for generation of MGE cells. We applied 1000 ng/ml of SHH at an early timepoint to pattern hPSCs to nearly pure MGE progenitors. These progenitors can be further differentiated into cells that are electrophysiologically functional with 40% cholinergic neurons and 50% GABA interneurons in vitro.[19]
In order to further determine whether the hPSC-derived MGE progenitors are functional in vivo, we generated a mouse model with learning and memory deficits by using mu p75-SAP (Cat. #IT-16). This targeted toxin consists of an affinity-purified rabbit polyclonal antibody specific to the mouse low affinity nerve growth factor receptor (NGFr) conjugated to the ribosome-inactivating protein, saporin. We injected 1.5 μg of mu p75-SAP into the medial septum; another group of mice were injected with artificial cerebral spinal fluid (aCSF) as sham lesion control. Two weeks after injection we checked the expression levels in the medial septum of cholinergic neurons and GABA interneurons: almost all the cholinergic neurons were killed by mu p75-SAP; and parvalbumin (PV, one of GABA interneuron subtypes) neurons were also decreased.* When tested in the Morris water maze, the learning and memory ability of lesioned mice was significantly decreased as compared to sham mice.
After one month post-lesion, we injected MGE progenitors into two sides of the hippocampus that are in the area of the medial septal neurons. VSP (ventral spinal progenitors) were injected as a cellular transplantation control, and aCSF as a surgery control. Human MGE progenitors differentiated into cholinergic neurons and GABA interneurons in the mouse hippocampus and formed functional connections with host neurons as tested by slice electrophysiology and immunostaining. Six months after transplantation, using the Morris water maze test, the group receiving MGE progenitors had significantly increased learning and memory ability, while control groups did not learn well. We conclude that MGE cells have a function in vivo to ameliorate learning and memory deficits in lesioned mice.
*Editor’s Note: In order to establish this model properly, a higher dose than usual of mu p75-SAP was used which caused some non-specific damage. For standard lesioning applications, ATS recommends that a control conjugate (non-targeted agent conjugated to saporin) be used in experiments to guarantee there is no non-specific cell death.
References: (back to top)
- Sussel L, Marin O, Kimura S, Rubenstein JL. (1999) Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126:3359-3370.
- Oliveira AA, Jr., Hodges HM. (2005) Alzheimer’s disease and neural transplantation as prospective cell therapy. Curr Alzheimer Res 2:79-95.
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237-1239.
- Pang KC, Jiao X, Sinha S, Beck KD, Servatius RJ. (2010) Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: Effects on proactive interference. Hippocampus 21:835-846.
- Dwyer TA, Servatius RJ, Pang KC. (2007) Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci 27:299-303.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145-1147.
- Takahashi K, Tanabe K, Ohnuki M, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861-872.
- Zhang SC. (2006) Neural subtype specification from embryonic stem cells. Brain Pathol 16:132-142.
- Yamanaka S. (2009) A fresh look at iPS cells. Cell 137:13-17.
- Liu Y, Zhang SC. (2010) Human stem cells as a model of motoneuron development and diseases. Ann N Y Acad Sci 1198:192-200.
- Li XJ, Hu BY, Jones SA, et al. (2008) Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells 26:886-893.
- Xi J, Liu Y, Liu H, Chen H, Emborg ME, Zhang SC. (2012) Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells 30:1655-1663.
- Yan Y, Yang D, Zarnowska ED, et al. (2005) Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23:781-790.
- Li XJ, Du ZW, Zarnowska ED, et al. (2005) Specification of motoneurons from human embryonic stem cells. Nat Biotechnol 23:215-221.
- Ma L, Hu B, Liu Y, et al. (2012) Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 10:455-464.
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. (2007) Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci 27:9682-9695.
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. (1998) Regionalization of the prosencephalic neural plate. Ann Rev Neurosci 21:445-477.
- Wilson SW, Rubenstein JL. (2000) Induction and dorsoventral patterning of the telencephalon. Neuron 28:641-651.
- Liu Y, Weick JP, Liu H, et al. (2013) Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat Biotechnol 31:440-447.