Contributed by Kiyoshi Ariizumi, Hideo Akiyoshi, Jin-Sung Chung, Mizuki Tomiharu, Ponciano D. Cruz Jr.
Department of Dermatology, The University of Texas Southwestern Medical Center and Dermatology Section (Medical Service),
Dallas Veterans Affairs Medical Center, Dallas, TX
T lymphocyte activation is regulated by stimulatory and inhibitory signals transduced by binding of T cell receptors to corresponding ligands on antigen-presenting cells (APC). Stimulatory receptors tend to be present constitutively even on resting T cells, whereas many inhibitory receptors require activation for expression. [1] Thus, inhibitory receptors may serve as a marker for the functional state of T cells.
We discovered a novel inhibitory pathway composed of the APC receptor DC-HIL and its exclusive T cell ligand, syndecan-4 (SD-4). DC-HIL specifically recognizes particular structures of heparan sulfate on SD-4 peculiar to T cells. SD-4 is expressed by activated (but not resting) T cells, including effector/memory CD4+ and CD8+ T cells. Infusion of soluble DC-HIL into mice inhibits the DC-HIL/SD-4 pathway, and results in enhanced immune responses. The current report addresses the hypothesis that depleting SD-4+ T lymphocytes using DC-HIL conjugated to a toxin will suppress elicitation of a T cell-mediated inflammatory response.
We biotinylated and conjugated soluble DC-HIL receptor or control Fc alone (IgG-SAP) to Streptavidin-ZAP (streptavidin conjugated to saporin; Cat. #IT-27), and showed that DC-HIL-SAP binds specifically to activated T cells, is internalized by these cells, and inhibits T cell proliferation in a SD-4- specific manner. These results document that DC-HIL-SAP selectively kills SD-4+ activated T cells.
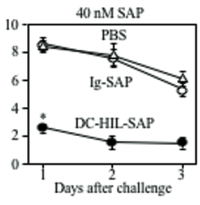
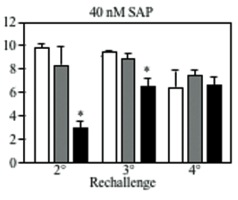
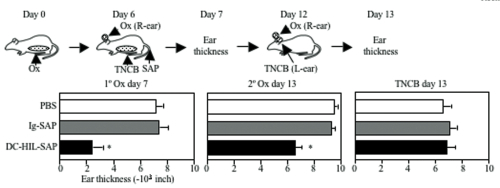
We next examined the effect of DC-HIL- SAP on an ongoing contact hypersensitivity (CH) response, which is an established model of a delayed T cell-mediated response. Mice were sensitized to a contact allergen oxazolone (Ox) on abdominal skin (day 0), then challenged with Ox on ear skin (day 6). Mice were injected i.v. with DC-HIL-SAP, IgG-SAP (control conjugate), or PBS 3 h prior to challenge (Fig. 1). PBS-injected mice developed strong ear swelling, whereas DC-HIL-SAP- injected mice exhibited markedly reduced ear swelling by 80%. IgG-SAP had no effect. Our DC-HIL-SAP concentration was optimal since 20 nM caused 50% suppression, whereas 80 nM produced 80% reduction (similar dose of IgG-SAP causing increased toxicity). Histologic examination of Ox-painted ear skin in DC-HIL-SAP-injected mice revealed less thick ears and fewer infiltrating leukocytes (Fig. 2). Injection of DC-HIL-SAP following Ox challenge also reduced CH response. The unresponsive state to Ox lasted for 3 weeks (Fig. 3), even as these same mice were able to mount effective CH response against another contact allergen 2,4,6- trinitrochlorobenzene (TNCB) (Fig. 4).
These results indicate that a single infusion of DC-HIL- SAP efficiently blocks elicitation of an established immune response that lasts for 3 weeks and is restricted to the antigen introduced at the time of treatment.
We also examined the ability of DC-HIL-SAP to deplete SD-4+ T cells in immunized mice. Two days after challenging sensitized mice treated with DC-HIL-SAP or controls, SD-4+ T cells in Ox-painted ear skin or in draining lymph nodes (DLN) were counted by immunofluorescent staining (Fig. 5A) or by flow cytometry (Fig. 5B), respectively. There were none- to-very few T cells in untreated skin, but many CD4+ and CD8+ T cells in Ox-painted skin, almost all of which were SD-4+ (Fig. 5A). Numbers of CD4+ and CD8+ T cells in skin of mice injected with IgG-SAP were similar to those of mice treated with PBS, whereas both were reduced markedly following DC-HIL-SAP infusion. In DLN, infusion of DC- HIL-SAP depleted by 40% CD4+ and CD8+ T cells. These results indicate that a single infusion of DC-HIL-SAP depletes SD-4+ T cells in the inflamed skin and DLN.
Our studies in mice indicate that SD-4 can be targeted using toxin-bearing DC-HIL to alleviate a cutaneous inflammatory response that may find applications in many human disease states. The targeted nature (SD-4+ T cells) of this treatment may hold special advantage with respect to safety.
References/Footnotes: (back to top)
- T cell expression profiles of these receptors overlap but are disparate; cytotoxic T Lymphocyte antigen-4 (expressed by almost all recently activated T cells), programmed cell death-1 (restricted to effector T cells), B and T lymphocyte attenuator and T cell immunoglobulin mucin 3 (expressed preferentially by Th1 cells). Moreover, sustained high-level of programmed cell death-1 expression is a marker for T cells undergoing exhaustion in chronic viral infections and in cancer.
- Chung J-S, Sato K, Dougherty I, Cruz PD Jr, Ariizumi K. DC-HIL is a negative regulator of T cell activation. Blood 109:4320-4327, 2007.
- Akiyoshi H, Chung J-S, Tomihari M, Cruz PD Jr, Ariizumi K. Depleting syndecan-4+ T lymphocytes using toxin-bearing DC-HIL: A new
opportunity for treating activated T cell-driven disease. J Immunol April 2010.