Contributed by Kevin C.H. Pang, Xilu Jiao and Richard J. Servatius
Neuroscience Dept, New Jersey Med Sch – UMDNJ and VA Med Ctr – East Orange, NJ
The medial septum and diagonal band of Broca (MSDB) provide a major afferent pathway to the hippocampus.[1] Cholinergic and GABAergic neurons are the main components of this pathway, but glutamatergic and peptidergic neurons also contribute.[1-5] Damage of MSDB neurons or the septohippocampal pathway impairs learning and memory, and disrupts hippocampal theta rhythm.[6-7] Although the role of cholinergic septal neurons in learning and memory and hippocampal function is well studied, little is known about the contribution of noncholinergic neuronal populations. In previous studies, intraseptal kainic acid was found to preferentially damage noncholinergic MSDB neurons, to drastically reduce hippocampal theta rhythm, and to impair spatial reversal learning.[8-11] The current study was performed to assess the cytotoxic and behavioral effects of a novel GABAergic toxin.
GAT1-SAP (Cat. #IT-32) is a rabbit antibody to the GABA transporter GAT1 that is conjugated to the ribosome-inactivating protein, saporin. The behavioral effect of GAT1-SAP administration into the MSDB was assessed in two versions of the water maze and in an active avoidance task. In one version of the water maze, the escape platform remained in the same location every day (reference memory). In the second version, the escape platform was moved to a new location every day (working memory). For this study, rats were tested in the reference memory version prior to the working memory version. Another group of rats was assessed in a lever press avoidance task. In this task, a tone (warning signal) was presented at the start of a trial. If a lever press occurred less than sixty seconds from the start of the trial, the warning signal was terminated and an intertrial interval signaled by a flashing light began with a duration of three minutes; this constituted an avoidance response. If a lever press did not occur prior to sixty seconds from the start of the trial, intermittent foot shock (0.5-second shock every three seconds) was delivered and continued until a lever press or five minutes occurred. Either lever press or the end of the five-minute duration resulted in termination of the warning signal and foot shock and the start of the intertrial interval. A session consisted of twenty trials, each session occurring three times a week. Acquisition of the lever press avoidance procedure was followed by extinction sessions in which the foot shock was not delivered. Following behavioral testing, immunocytochemistry was performed to assess the damage produced by GAT1-SAP.
Intraseptal GAT1-SAP reduced the number of GABAergic septohippocampal neurons containing the calcium-binding protein, parvalbumin (Figure 1).[2] The number of cholinergic neurons, identified by the presence of choline acetyltransferase, was not markedly affected. Behaviorally, GAT1-SAP did not alter performance on the reference memory version of the water maze (data not shown), but did impair learning in the working memory version (Figure 2). Rats treated with GAT1-SAP returned to the original platform location (location during reference memory phase) more often than rats with sham surgery (Figure 2; main effects of Trials and Treatment: p < .05). GAT1-SAP rats also tended to return to the platform location of the previous day more often than sham controls (data not shown; main effect of Treatment: p = .055). The pattern of results was similar to that following MSDB damage with kainic acid.[8] In the avoidance procedure, GAT1-SAP did not alter acquisition (Figure 3A) but impaired extinction of the avoidance response (Figure 3B; main effect of Trial and Trial X Treatment interaction: p < .001). Overall, the results support the view that damage of GABAergic MSDB neurons enhances proactive interference and perseveration, possibly by interfering with hippocampal theta rhythm.[12]
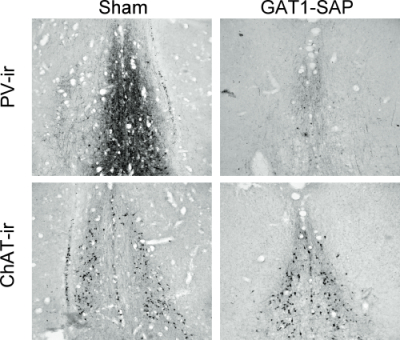
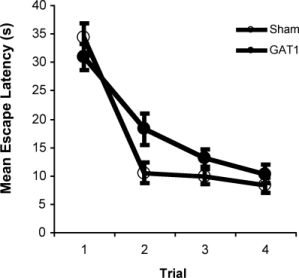
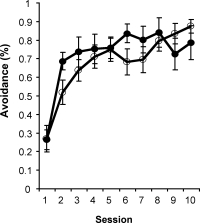
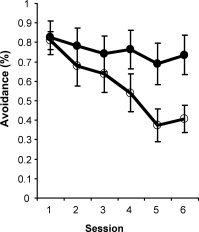
Figure 3. Extinction (B) but not acquisition (A) of an active avoidance response was impaired by intraseptal GAT1-SAP.
References: (back to top)
- Amaral DG, Kurz J (1985) An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol 240(1):37-59.
- Freund TF (1989) GABAergic septohippocampal neurons contain parvalbumin. Brain Res 478(2):375-381.
- Kiss J, Borhegyi Z, Csaky A, Szeiffert G, Leranth C (1997) Parvalbumin-containing cells of the angular portion of the vertical limb terminate on calbindinimmunoreactive neurons located at the border between the lateral and medial septum of the rat. Exp Brain Res 113(1):48-56.
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S (2003) Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol 551(Pt 3):927-943.
- Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E (2005) Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 58(3):151-164.
- Kesner RP, Crutcher KA, Measom MO (1986) Medial septal and nucleus basalis magnocellularis lesions produce order memory deficits in rats which mimic symptomatology of Alzheimer’s disease. Neurobiol Aging 7(4):287-295.
- Winson J (1978) Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201(4351):160-163.
- Dwyer TA, Servatius RJ, Pang KC (2007) Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci 27(2):299-303.
- Malthe-Sørenssen D, Odden E, Walaas I (1980) Selective destruction by kainic acid of neurons innervated by putative glutamergic afferents in septum and nucleus of the diagonal band. Brain Res 182(2):461-465.
- Pang KC, Nocera R, Secor AJ, Yoder RM (2001) GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus 11(6):814-827.
- Yoder RM, Pang KC (2005) Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus 15(3):381-392.
- Hasselmo ME (2005) What is the function of hippocampal theta rhythm?–Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15(7):936-949.
