Contributed by Neelima Chauhan, Dept Anesthes/Anat & Cell Bio, Univ Illinois-Chicago;
J. Brown VA Med Ctr Chicago, Chicago, IL and winner of the 2006 SfN Poster Award for work using ATS products.
Today’s Alzheimer’s disease (AD) research lacks a “complete” model that would represent both plaque and tangle pathology together with correlative memory deficits. The currently available transgenic model that includes APP/PS1/tau mutations does not truly represent AD because tangles observed in AD brain are independent of tau mutations. Subtly increased β-amyloid (Aβ) levels either due to familial mutations or sporadic causes, primarily signals pre-tangle cytopathology and degeneration of basal forebrain cholinergic neurons (BFCN) via deranged signaling of glygogen synthase kinase 3-beta (GSK3β)-, protein kinase A (PKA)-, and extracellular signal-regulated kinase (ERK2) of the ERK-mitogen-activated protein kinase (MAPK) cascade. This leads to reduced phosphorylation of cAMP responsive element binding protein (CREB) that results in synaptic and memory deficits much earlier than the emergence of classic AD pathology. Thus, subtly elevated Aβ, together with BFCN deficits resulting from Aβ-induced deranged signaling, set up a vicious feedback loop to produce characteristic plaque and tangle pathology observed in AD.
Based on these facts, we wished to test if selective lesioning of BFCN during the early stages of amyloid build-up exacerbate tau phosphorylation and produce tangle-like inclusions in transgenic mice with APP mutations. We produced selective immunotoxic lesions of BFCN by injecting the BFCN-specific cholinergic immunotoxin, mu p75-SAP (Cat. #IT-16) which is known to specifically target p75-expressing BFCN. This specific targeting agent was administered intracerebroventricularly in TgCRND8 mice harboring Swedish (KM670/671NL) and Indiana (V717F) mutations. The resulting model exhibited tangle-like inclusions, provoked already existing plaque pathology, and worsened impaired behavioral deficits. The combination of the transgenic mice treated with the immunotoxin provides a powerful tool for understanding AD progression.
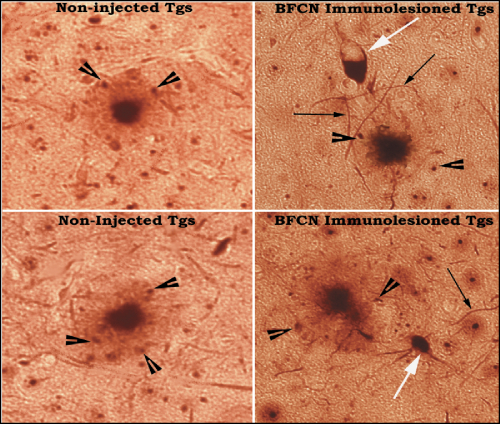
Black arrowheads: plaque-associated hyperphosphorylated neurites;
Black thin arrows: hyperphosphorylated neuropil threads in immunotoxin injected Tg brain (Right panel);
White arrow: “Tangled” neuron in the vicinity of plaque showing intraneuronal phosphorylated tangle-like inclusion in immunotoxin injected Tg brain (Right panel).
Note the absence of hyperphosphorylated neuropil threads and “Tangled” neuron in the vicinity of plaque in non-injected Tgs (Left panel).
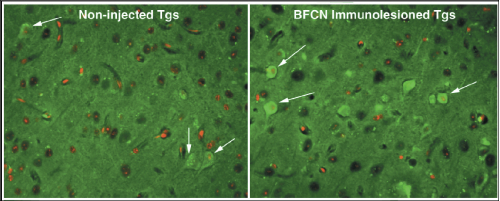
Left panel, white arrows: Cortical pyramidal neurons of non-injected Tg brain showing occasional punctate immunoreactivity for AT-8 (a marker protein for tangles) indicating “subtle” tau phosphorylation in untreated Tg brain.
Right panel, white arrows: Cortical pyramidal neurons of immunotoxin-injected Tg brain showing strong immunoreactivity for AT-8 (a marker protein for tangles) indicating the presence of “tangle-bearing” neurons in immunotoxin-injected Tg brain.
