Contributed by William Truitt, PhD, Department of Cell Biology, Neurobiology, and Anatomy, Neuroscience Graduate Program, University of Cincinnati, College of Medicine, Post Office Box 670521, Cincinnati, OH 45267-0521, USA.
Previously we demonstrated the existence of a spinothalamic pathway in the male rat where neural activation is specifically induced by ejaculation.1 This pathway includes the parvocellular subparafascicular thalamic nucleus (SPFp) and projection neurons located in the lumbar spinal cord. These lumbar spinothalamic (LSt) neurons are located in lumbar segments 3 and 4 and can be identified by neural peptide content including galanin. To test the behavioral significance of these lumbar spinothalamic (LSt) neurons, effects of lesions of the LSt population on sexual behavior were investigated.2
Methodology: LSt neurons are sparsely distributed lateral to the central canal in lamina X and in the medial portion of lamina VII of L3 and L4, and are difficult to lesion by traditional methods. We thus identified a membrane target located on the LSt neurons. It was demon- strated that 93% of LSt neurons express neurokinin-1 receptor (NK-1r) and conversely 85% of NK-1r containing cells in the area surrounding the central canal at L3-4 express galanin (Fig. 1). We therefore used the targeted toxin SSP-SAP (a stable form of Substance P conjugated to Saporin) for specific ablation of LSt cells. A series of 6-8 bilateral 1-μl SSP- SAP injections (4 ng) was infused into the L3-4 spinal cord at the location of the LSt cells in male rats. Control animals were injected with non-conjugated equimolar concentrations of SAP (Saporin). Sexual behavior was first tested 10 days after surgery, and during 5 subsequent twice-weekly tests. Following the final behavior test, animals were perfused and spinal cord tissue was immuno-processed for galanin, NK-1r, or neuronal marker N (NeuN). Labeled cells were counted in a standard area surrounding the central canal of L3-4 sections representative of the location of LSt cells.
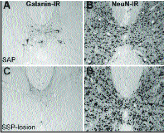
SSP-SAP treatment resulted in two groups, rats with complete lesions of LSt neurons (SSP-les; defined by less than 1/3 of the number of LSt cells observed in untreated rats), and rats with incom- plete or misplaced lesions (SSP-il). No lesions were present in SAP-treated males (SAP). SSP-les animals had fewer galanin-immunoreactive (IR; Fig. 2) and NK-1r-IR neurons than SSP-il or SAP animals. Despite the severe reduction in LSt neurons in SSP-les rats, there was no overall reduction in numbers of NeuN-IR cells (Fig. 2), indicating the selectivity of the lesions. Furthermore, the lesions were restricted to the area surrounding the central canal and did not affect the number of NK-1r expressing neurons in the dorsal horn.
LSt lesions had dramatic effects on sexual behavior. Lesions completely disrupted display of ejaculatory behavior in SSP-les males and seminal plugs were uniformly absent upon examination of the female partner throughout the testing session. In contrast, SSP-il and SAP males continued to ejaculate regularly following surgery. Furthermore, ablation of LSt neurons selectively blocked ejaculatory behavior without affecting other components of sexual behavior. SSP-les animals did not differ from the SSP-il or SAP animals in number of mounts or intromissions (Fig. 3).

It is well established that ejaculation is a reflex and ejaculatory reflexes remain intact when control by supra- spinal sites is eliminated, suggesting the existence of a spinal ejaculation generator. However, the anatomical site of such an ejaculation generator is yet unknown. Our data suggest that LSt cells form a critical component of the ejaculation generator.
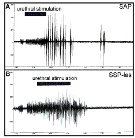
To further test this hypothesis, effects of LSt cell lesions on expression of ejaculatory reflexes were investigated. We utilized a model that reliably mimics ejaculatory responses in anesthetized, spinalized male rats, i.e. the urethro- genital reflex model.3 In short, urethral stimulation induces characteristic organized bursting patterns of peripheral nerves, smooth muscles and striated muscles, including the bulbocavernosus muscle (BCM). In the present study, urethrogenital reflexes were investigated in males with complete and incomplete LSt lesions, and SAP controls. LSt cell lesions were performed using SSP-SAP as described above and BCM bursting patterns were monitored. LSt lesions dramatically disrupted ejaculatory reflexes. In SSP-les males, urethral stimulation resulted in asynchronous, low amplitude, short bursts of the BCM (Fig. 4B). In contrast, urethral stimula- tion resulted in a characteristic organized and synchronized series of 8 to 12 BCM bursts in SSP-il and SAP control males (Fig. 4A). Together these data demonstrate that LSt cells play a pivotal role in control of ejaculatory reflexes and behavior.
REFERENCES
- Truitt WA, Shipley MT,Veening JG, Coolen LM (2003) J Neurosci 23:325-331.
- Truitt WA, Coolen LM (2002) Science 297(5586):1566-1569.
- McKenna KE, Chung SK, McVary KT (1991) Am J Physiol 261(5 Pt 2) R1276-R1285.
Related product information: SSP-SAP (Cat. #IT-11)
