One of the more common methods of using Streptavidin-ZAP is to couple the complex with biotinylated antibodies. However, there are also many instances of biotinylated ligands, peptides, and even biotinylated major histocompatibility complex (MHC) tetramers in immunology fields.
B-Cell Targeting. An example of utilizing Streptavidin-ZAP to study ligands that target CD22 were shown by Collins et al. [1]. CD22 is a potential target for immunotherapy of B-cell lymphomas. The authors examined the equilibrium between CD22 and the cis and trans forms of its ligands using high affinity sialoside probes. They also demonstrated that a biotinylated probe specific for CD22, when combined with Streptavidin-ZAP, can eliminate several different lymphoma cell lines.
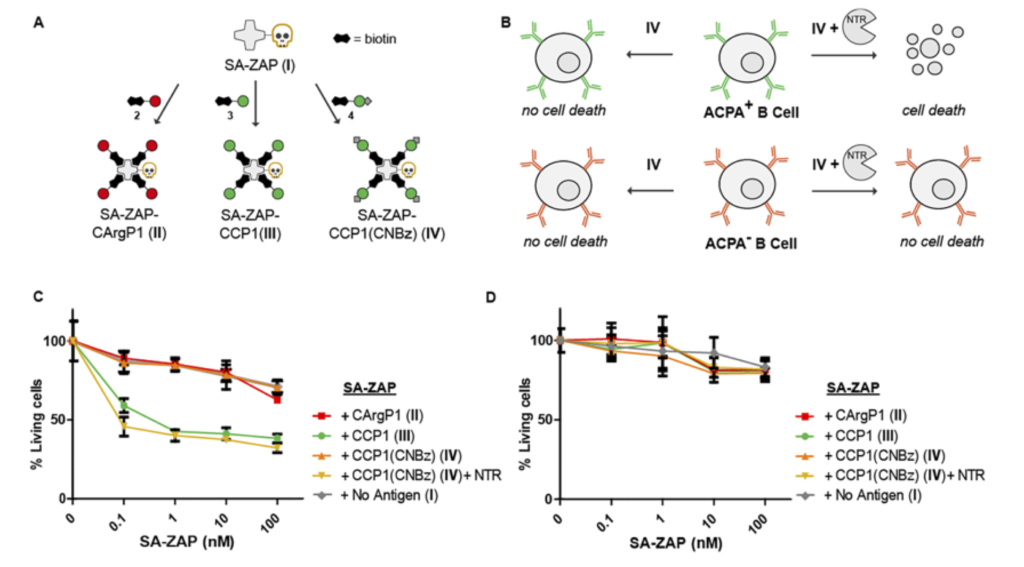
Figure 5. Selective cytotoxicity by enzymatic activation of CCP1-SA-ZAP conjugates. Schematic representation of streptavidin-ZAP bound to the CXP1 peptides (A) and the expected toxicity of the different SA-ZAP conjugates to ACPA expressing B cells (B). Percentage of living ACPAexpressing B cells (C) and TT-specific B cells (D) after 4 days of treatment with antigen-toxin conjugates [2].
Rheumatoid arthritis is a chronic disease that is accompanied with anti-citrullinated protein antibodies (ACPA) produced by autoreactive B cells. A study in 2018 used a synthesized cyclic citrullinated peptide (CCP) antigen suitable for B-cell receptor binding and demonstrated that binding by ACPA was impaired upon manipulation of the residue [2]. The data were generated using biotinylated CCP mixed with Streptavidin-ZAP in cell viability assays. The results marked an important step towards antigen-selective B-cell targeting in general, and more specifically in rheumatoid arthritis.
T-Cell Targeting. An example of using Streptavidin-ZAP to deplete specific T cells was published in 2010; Akiyosi et al. used the dendritic cell-associated heparan sulfate proteoglycan- dependent integrin ligand (DC-HIL) as the targeting agent. DC-HIL is exclusively associated with syndecan-4 (SD-4) which is expressed on some activated T cells [3]. A similar study was done with Sézary syndrome cells that overexpress syndecan-4 [4].
Hess et al. investigated whether pathogenic T cells could be depleted via Streptavidin-ZAP coupled to MHC class I tetramers to kill antigen-specific CD8+ T cells [5]. Their work showed the therapeutic potential for using cytotoxic tetramers to eliminate specific T cells. This same strategy was employed in vivo to delay diabetes in non-obese diabetic mice [6]. The Hess group also used biotinylated peptide-MHC class I tetramers with Streptavidin-ZAP to selectively deplete a population of alloreactive T cells in mice to determine that toxic tetramer administration prior to immunization increased survival of cognate peptide-pulsed cells in an in vivo cytotoxic T lymphocyte assay and reduced the frequency of corresponding T cells [7]. More research towards T-cell depletion utilizing Streptavidin-ZAP and biotinylated MHC tetramers came from Sims et al. where they found that following a significant transient depletion of cells, the population rebounded and reached a higher percentage of total CD8+ T cells than before the depletion. This research provides helps further understanding of the ‘flexibility and turnover’ of these cells [8].
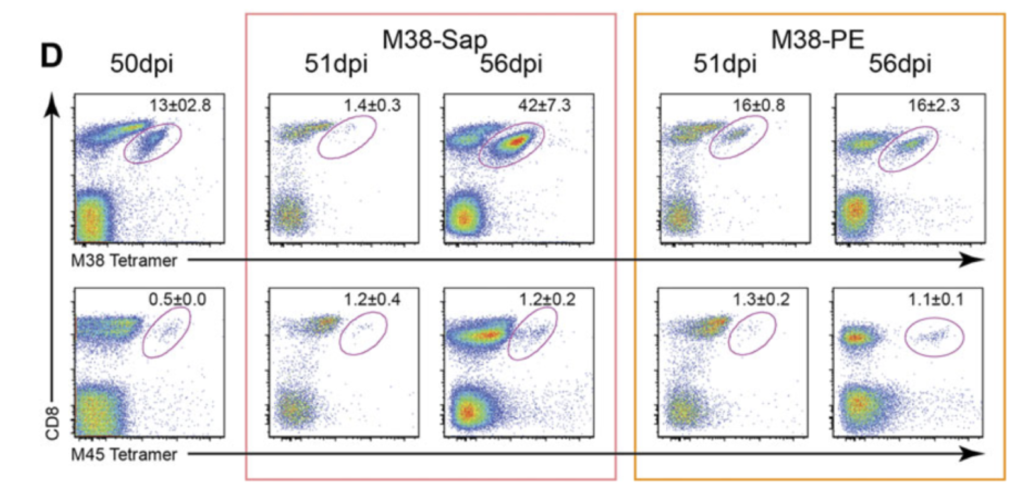
Figure 1. Frequency and function of MCMV-specific CD8+ T cells and depletion of M38- specific CD8+ T cells. C57BL/6 mice were infected i.v. with 1 x 106 pfu MCMV. D) Representative flow cytometry plots of M38-specific CD8+ T cells and M45-specific CD8+ T cells 50 days after MCMV infection, 1 and 6 days after either M38-tetramer-saporin (red box) or M38-tetramer-PE injection (orange box). Data are shown as mean ± SEM and are from one representative plot out of 6/group) [8].
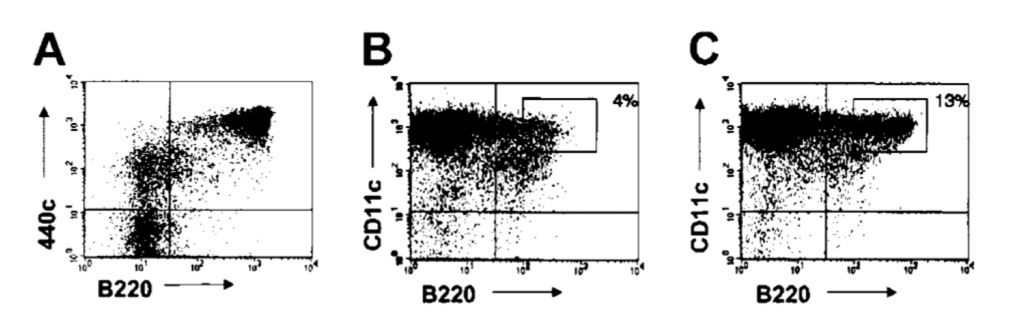
Figure 7. Sequential treatment with mAb 440c and saporin depletes IPCs in vitro. (A) Expression of 440c on bone marrow cells cultured in FLT3-L for 10 days. CD11c cells were excluded from the gate. 440c is highly expressed on B220 cells, which represent fully developed IPCs. (B-C) Depletion of CD11c /B220 cells from FLT3-L–derived bone marrow IPCs by treatment with 440c-saporin. Cells were stained on ice with biotinylated-440c (B) or a biotinylated control antibody (C), followed by avidin-saporin, cultured for an additional 36 hours, and analyzed for residual presence of CD11c /B220 cells. The percentage of gated cells is indicated. Results are representative of 3 separate experiments [10].
Other Applications. Outside of T cells and B cells, Streptavidin-ZAP has also been used to deplete dendritic cells (DC). Alonso et al. depleted inflammatory DCs with biotinylated anti- CD209 via intravenous injection in a mouse animal model of induced inflammatory DC formation [9]. The authors suggest that the depletion of inflammatory DCs could be useful in understanding inflammatory diseases such as psoriasis. Depletion of natural interferon-producing cells (IPCs) was demonstrated with an IPC-specific biotinylated antibody in vitro [10].
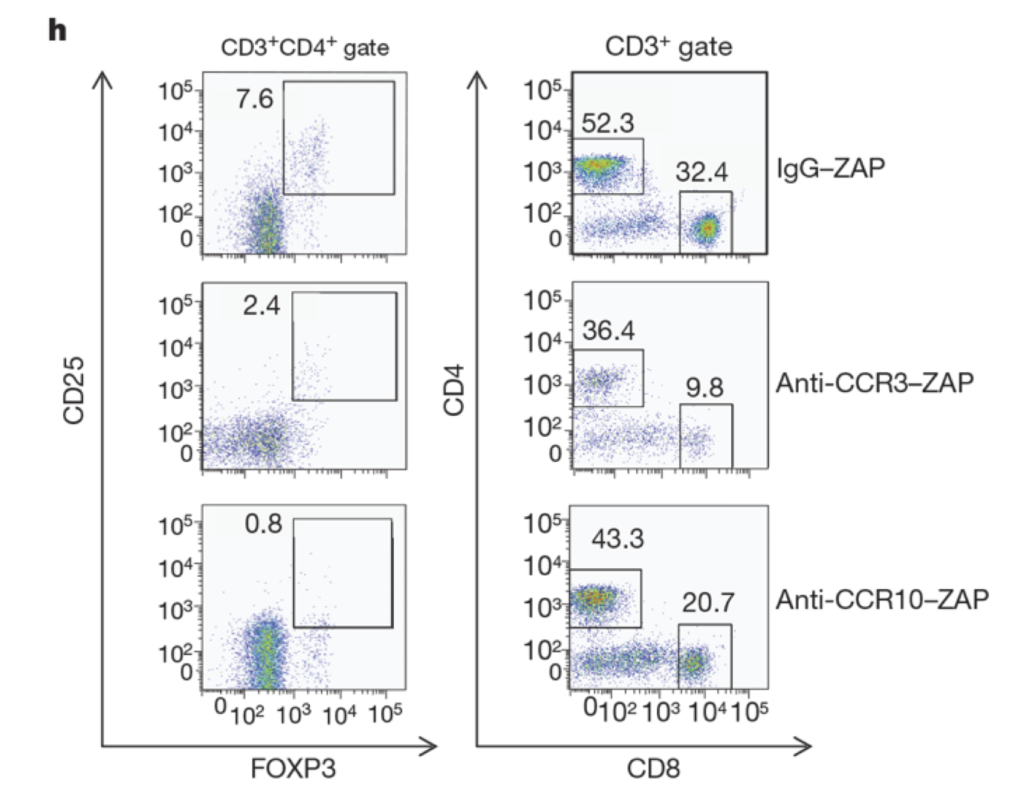
Figure 3. CCL28 promotes tumour growth through attracting CCR101 Treg cells. h) CCR10 depletion eliminates most CD41CD251FOXP31 cells but not CD81 T cells, as determined by flow cytometric analysis. CCR3 depletion eliminates both populations (numbers inside plots refer to the boxed areas: left column, % Treg cells; right column, % CD31CD41 cells (left) and % CD31CD81 cells (right)).or M38-tetramer-PE injection (orange box). Data are shown as mean ± SEM and are from one representative plot out of 6/group) [8].
Facciabene et al. made immunotoxins with Streptavidin-ZAP to biotinylated antibodies for CCR10 and CCR3 (Anti-CC10-Saporin and Anti-CCR3-Saporin). They investigated whether a direct link between tumor hypoxia and tolerance occurs through the recruitment of regulatory cells [11]. Their findings showed that peripheral immune tolerance and angiogenesis programs are closely connected and cooperate to sustain tumors.
References
- Collins BE, Blixt O, Han S, Duong B, Li H, Nathan JK, Bovin N, Paulson JC (2006) High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol 177(5):2994-3003. doi: 10.4049/jimmunol.177.5.2994 PMID: 16920935
- Lelieveldt LPWM, Kristyanto H, Pruijn GJM, Scherer HU, Toes REM, Bonger KM (2018) Sequential prodrug strategy to target and eliminate ACPA-selective autoreactive B cells. Mol Pharm 15(12):5565-5573. doi: 10.1021/acs.molpharmaceut.8b00741 PMID: 30289723
- Akiyoshi H, Chung JS, Tomihari M, Cruz PD, Jr., Ariizumi K (2010) Depleting syndecan-4+ T lymphocytes using toxin-bearing dendritic cell-associated heparan sulfate proteoglycan-dependent integrin ligand: A new opportunity for treating activated T cell-driven disease. J Immunol 184:3554-3561. doi: 10.4049/jimmunol.0903250 PMID: 20176742
Read the Targeting Trends Article. - Chung JS, Shiue LH, Duvic M, Pandya A, Cruz PDJ, Ariizumi K (2011) Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-beta on the cell surface. Blood 117(12):3382-3390. doi: 10.1182/blood-2010-08-302034 PMID: 21252093
- Hess PR, Barnes C, Woolard MD, Johnson MD, Cullen JM, Collins EJ, Frelinger JA (2007) Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood 109:3300-3307. doi: 10.1182/blood-2006-06-028001 PMID: 17179221
Read the Targeting Trends Article. - Vincent BG, Young EF, Buntzman AS, Stevens R, Kepler TB, Tisch RM, Frelinger JA, Hess PR (2010) Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J Immunol 184(8):4196-4204. doi: 10.4049/jimmunol.0903931 PMID: 20220085
- Hess SM, Young EF, Miller KR, Vincent BG, Buntzman AS, Collins EJ, Frelinger JA, Hess PR (2013) Deletion of naive T cells recognizing the minor histocompatibility antigen HY with toxin-coupled peptide-MHC class I tetramers inhibits cognate CTL responses and alters immunodominance. Transpl Immunol 29(1-4):138-145. doi: 10.1016/j.trim.2013.10.005 PMID: 24161680
- Sims S, Bolinger B, Klenerman P (2015) Increasing inflationary T-cell responses following transient depletion of MCMV-specific memory T cells. Eur J Immunol 45:113-118. doi: 10.1002/eji.201445016 PMID: 25331015
- Alonso M, Gregorio J, Davidson M, Gonzalez J, Engleman E (2014) Depletion of inflammatory dendritic cells with anti-CD209 conjugated to saporin toxin. Immunol Res 58:374-377. doi: 10.1007/s12026-014-8511-6 PMID: 24781193
- Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M (2004) A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-alpha. Blood 103(11):4201-4206. doi: 10.1182/blood-2003-09-3108 PMID: 14695235
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475:226-230. doi: 10.1038/nature10169 PMID: 21753853
