Screening targeting agents and their cell surface biomarkers for high specificity and rapid internalization via cell death and fluorescence.
Ancheta L, Bouajram R, Lappi DA (2018) Society for Neuroscience, San Diego, CA.
Abstract
Some of the most recent successes in the treatment of cancers or research into passive immunotherapies for neurodegenerative diseases, employ the use of antibodies. These treatments utilize antibodies that: 1) interfere with cell surface proteins responsible for tumor cell proliferation, or 2) act as immune checkpoint inhibitors, or 3) are re-engineered to allow transport of other molecules across the blood-brain barrier (BBB). There are a growing number of antibody and small molecule therapeutic candidates and this demands a quick and efficient technique to screen for biomarkers that internalize effectively upon binding. The method described provides for the efficient determination of internalization of cell surface biomarkers upon binding of antibodies or peptides. This one-step, robust method uses a targeting agent combined with both a fluorescent reporter and a cytotoxic payload. The construct that makes this method effective was formed by cross-linking a fluorescent reporter, in this case fluorescein (FITC) and streptavidin (SA) to the ribosome-inactivating protein, Saporin (ZAP). The conjugate used in screening potential therapeutics is a mixture of a biotinylated targeting agent mixed in a 1:1 molar ratio with FITC-labeled Streptavidinylated-Saporin (FITC-SA-ZAP). The method provides a definitive assay readout: fluorescence within 1 hour and cell death in 72 hours. This method is designed for rapid screening, in a quick and reproducible manner, for specificity and internalization in various cell types to explore suitability of candidates as therapeutics.
Figure 1
Introduction
According to the National Institutes of Health, in 2007 the U.S. incidence rate of brain and other CNS cancers among adults grew from 5.9 to 6.4 new cases per 100,000 persons; among children ≤19 years of age, it grew from 2.1 to 2.9 new cases per 100,000 persons. The higher incidence rates observed today are likely due, in part, to an improved ability to diagnose brain and other CNS tumors with advanced imaging tools and technologies. The importance of developing these new tools will play an important role in the on-going fight against cancer.
Streptavidin-ZAP (ATS; Cat. #IT-27) has become part of the landscape of methods in determining suitability of multiple targeting agents for binding and internalization of cell surface markers. It’s ability to assist researchers in evaluating potential candidates as targeting agents has especially displayed its relevance in cancer research.1,2 Building on that foundation, we describe here the synthesis and early characterization of a molecule that will act as a more inclusive screening tool allowing for both fluorescent detection and cell death via internalization of a cytotoxic payload.
Here, we present data from in vitro testing of three antibodies against p75NTR, 192- IgG (rat-specific monoclonal), mu p75 (mouse-specific polyclonal) and ME20.4 (mammalian-specific monoclonal). Nerve Growth Factor (NGF), the ligand for the p75 receptor, is a novel mediator of invasion of human glioma cells, but the role of the p75NTR in glioma proliferation is still unclear.3 Glioblastoma (GBM) is the most common primary malignant brain tumor, comprising 16% of all primary brain and central nervous system neoplasms.4
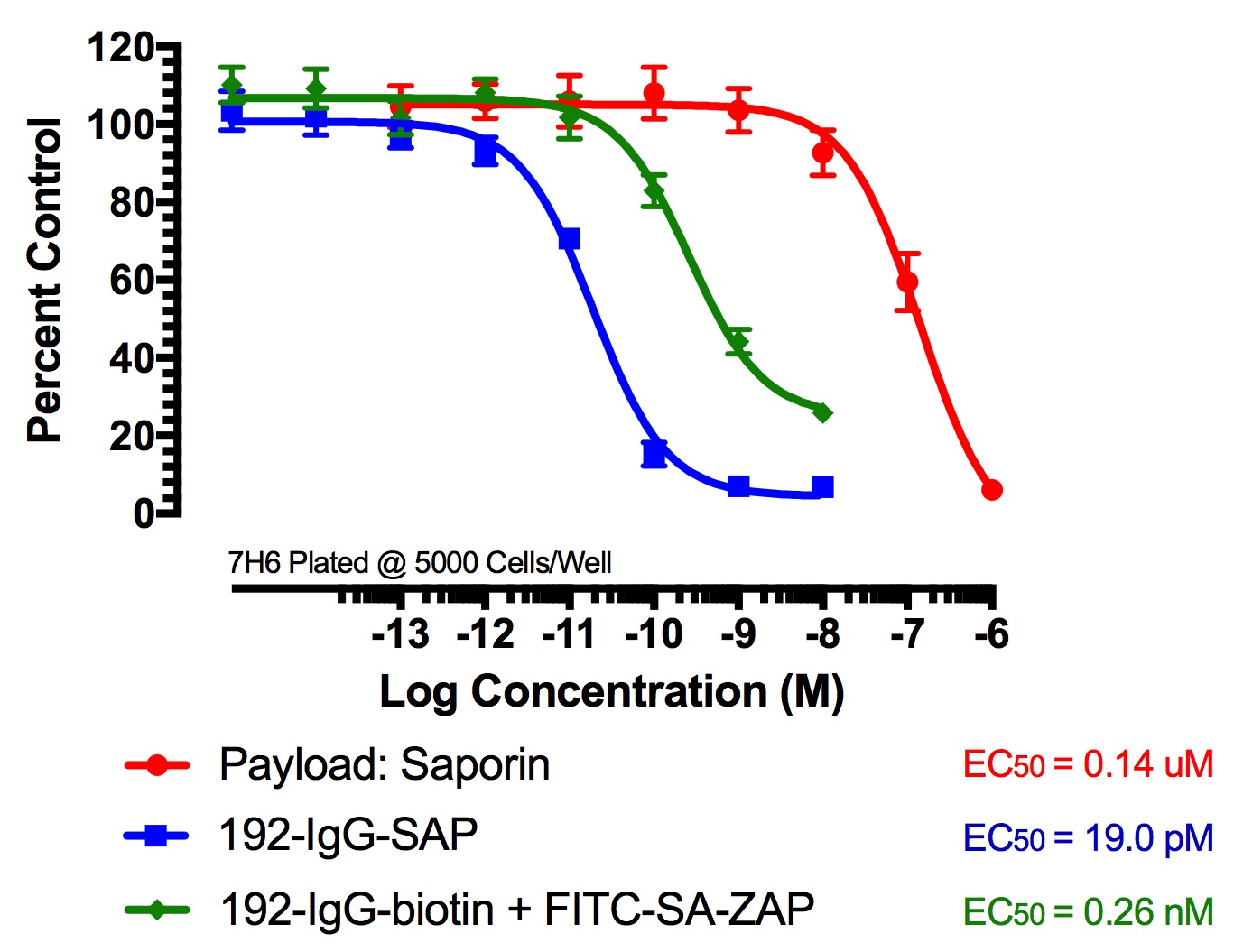
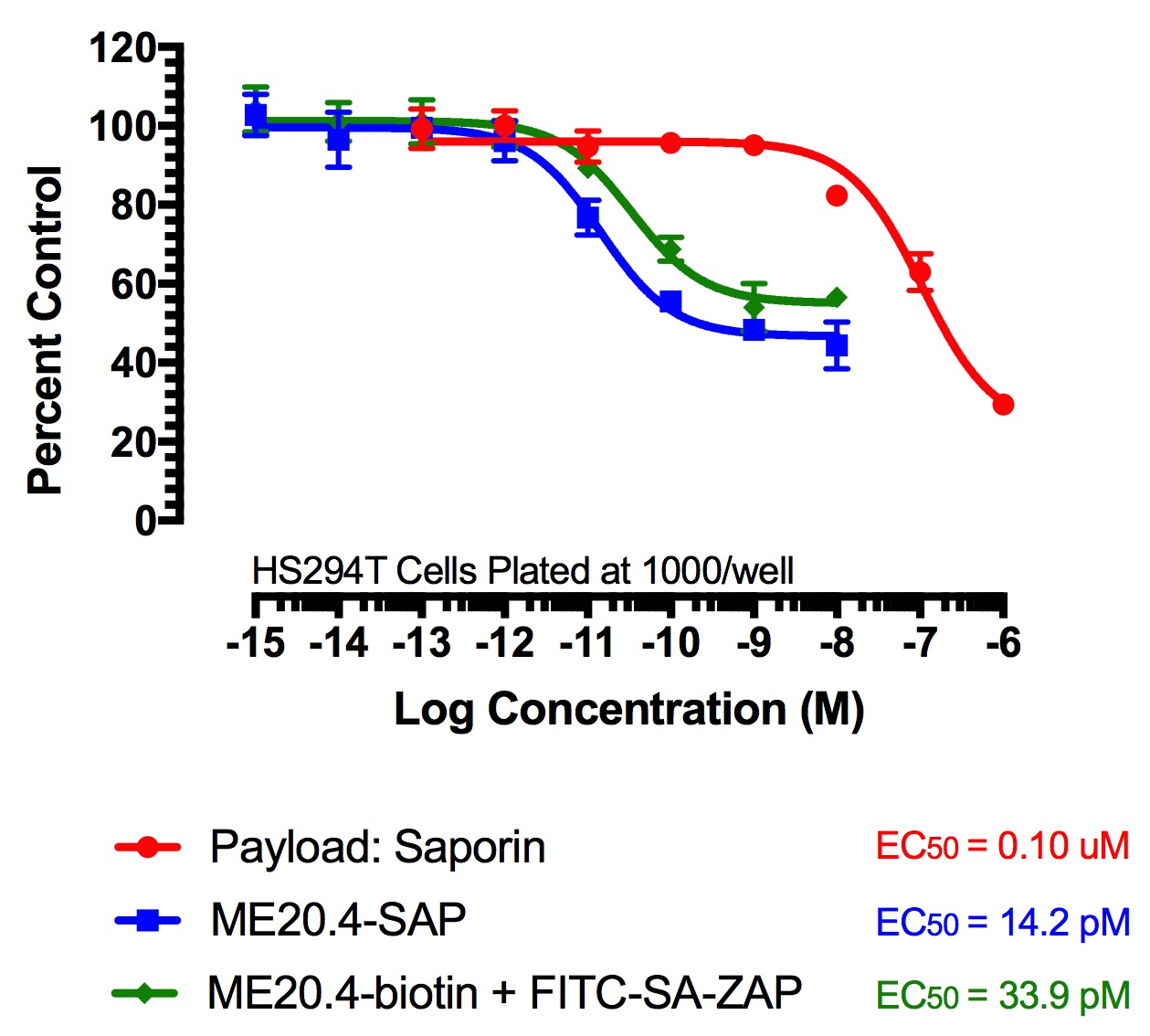
Figure 2
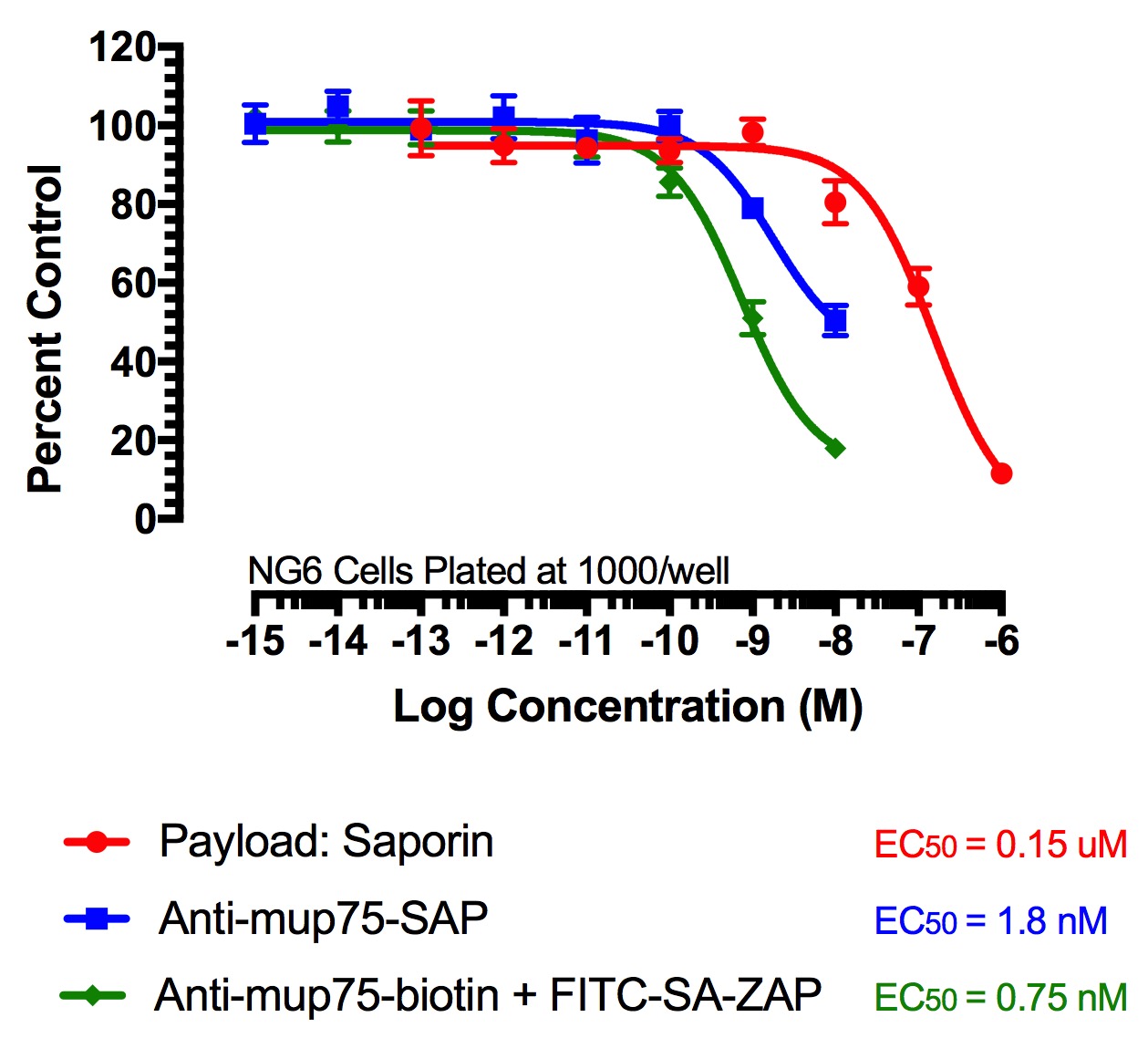
FITC-Streptavidin-Saporin (FITC-SA-ZAP) and biotinylated antibody demonstrates cytotoxicity via receptor-mediated endocytosis of cytotoxic payload. Treatments (FITC-SA-ZAP + biotinylated antibody, direct antibody-saporin conjugate, or control) were added in 10-µl doses with the highest concentration being 10 nM for the directly-linked conjugates and biotinylated antibodies and 1 µM for Saporin.
Methods
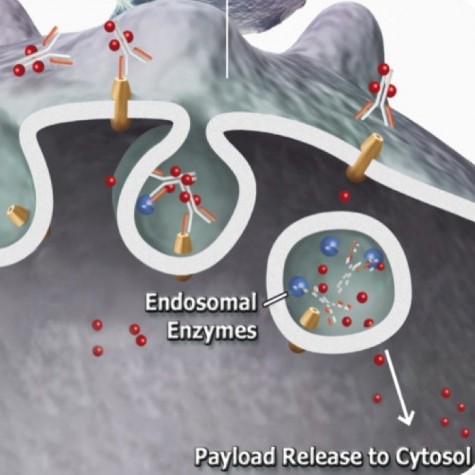
Two criteria must be established when using FITC-SA-ZAP, (1) biotinylation of the targeting agent and (2) the targeting agent recognizes a cell surface marker. This complex will fluoresce the cells recognized by the targeting agent and can subsequently eliminate the cells via receptor-mediated endocytosis.
Fig 2. Cytototoxicity Assay to Determine Binding and Internalization. Cells were plated into 96-well plates and incubated overnight at 37C to acclimate. Treatment of the cells occurred 24 hours later and unconjugated saporin and directly-linked conjugates (192-IgG-SAP, Anti-mu p75-SAP and ME20.4-SAP) were used as controls. The biotinylated antibodies were mixed at a 1:1 molar ratio with FITC-SA-ZAP. Developing reagents XTT/PMS or sulforhodamine-B were used to determine cell viability after treatment via absorbance.
Fig 3. Flow Cytometry to Detect Receptor Expression. One million cells were used for each test sample and were stained with 10 µg of biotinylated antibody mixed in a 1:1 molar ratio with FITC-SA-ZAP. Cells were treated for 1 hr at 4°C for cell-surface staining and were subsequently washed.
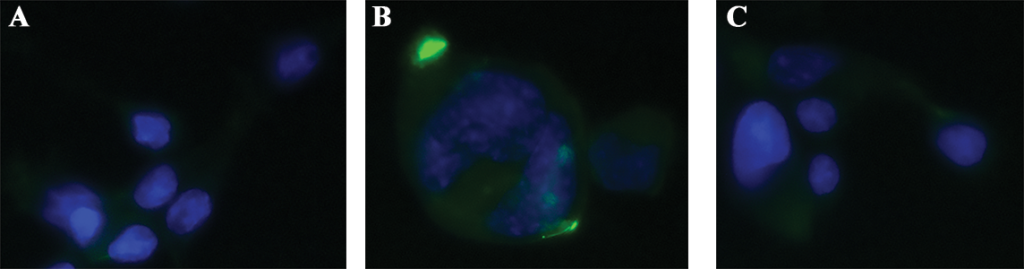
Fig 4. Immunocytochemistry to Confirm Binding and Receptor Expression. Cells were grown on glass cover slips treated with poly-L-lysine. Once cells reached confluency they were fixed and stained with biotinylated antibody mixed in a 1:1 molar ratio with FITC-SA-ZAP (green). Cells were incubated with antibody solution overnight and washed before subsequently stained with DAPI nuclear counter stain (blue). Images were acquired on a Leica DMIL fluorescent microscope using SPOT software.
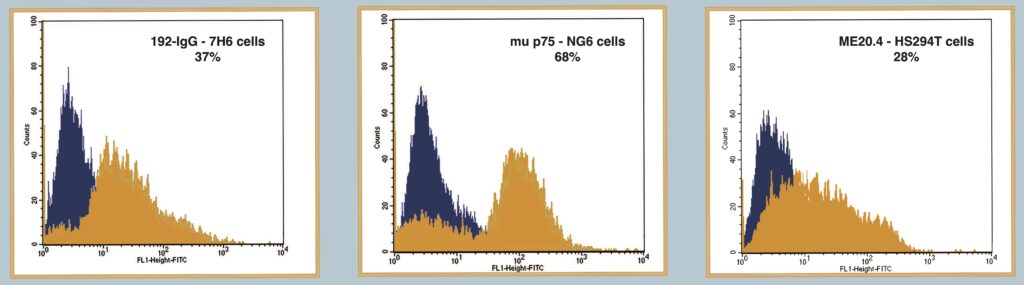
Figure 3
Demonstrates the use of FITC-SA-ZAP and biotinylated antibody in a flow cytometry application to detect receptor expression. The Orange peak shows expression of target receptor. Black peak shows control.
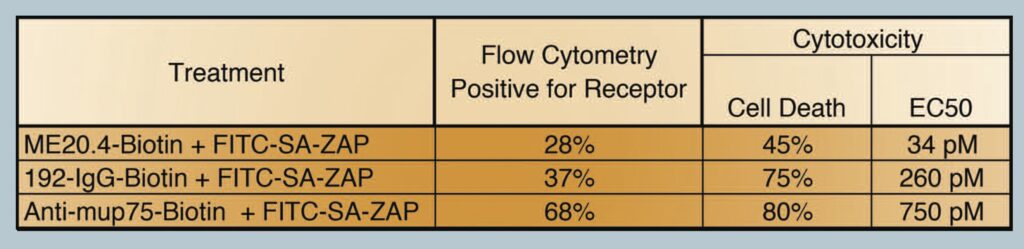
Figure 4
Immunocytochemical application for FITC-SA-ZAP mixed with biotinylated antibody confirming binding and expression.
A) FITC-SA-ZAP mixed with biotinylated 192 IgG antibody on 7H6 cells.
B) FITC-SA-ZAP mixed with biotinylated mu p75 antibody on NG6 cells.
C) FITC-SA-ZAP mixed with biotinylated ME20.4 antibody on HS294T cells.
Discussion
FITC-SA-ZAP was designed for the growing number of antibody and small molecule therapeutic candidates and the demands for a quick and efficient technique to screen for biomarkers that internalize effectively upon binding. We provided data of in vitro testing of 3 antibodies against p75NTR, 192-IgG (mouse monoclonal against rat), anti-mu p75 (rabbit polyclonal against mouse) and ME20.4 (mouse monoclonal against mammalian).
The first stage of screening consisted of visualizing the fluorescence levels seen from flow cytometry. This application demonstrated how quickly screening could be, taking only 1hr for the labeling of the cells. The second stage of screening involved the cytotoxicity seen via the internalization of the biotin-FITC-SA-ZAP molecule. These second stage results provided confirmation of not only the binding of the targeting agent, but its internalization, since saporin has no intrinsic mechanism of cell entry except by bulk-phase endocytosis at orders of magnitude higher concentration. As further confirmation of binding, ICC was performed with fluorescence visualized. A third method, not looked at in this study, is a potential application in which the targeted molecule could be tracked within a system using confocal microscopy.
From our results, we did not observe a direct correlation between the amount of cells positive for a receptor (flow cytometry) and the amount of cell death seen (cytotoxicity), i.e a 28% positive shift from flow cytometry did not translate into 28% of the cells being killed in a cytotox. These data did however provide a trend in which the flow cytometry data helped predict how well the targeting agent may work in the cytotox assay. Table 1. Shows the percentage of the cell population positive for the receptor versus the percentage of cell death via cytotox. Biotinylated ME20.4 performed the worst of the three antibodies on HS294T cells in flow cytometry (28%) and resulted in the lowest amount of cell death in cytotox on the same cells (45%). Biotinylated-anti-mu p75 performed the best in flow cytometry on NG6 cells (68%) and also showed the most amount of cell death in cytotox on the same cells (80%).
A main point that can be derived from these data is that regardless of what host the targeting agent was created in or what species the targeting agent was specific for, only one molecule was needed to perform a total of three applications providing evidence of affinity, specificity and internalization.
In comparison to having direct conjugates made with every antibody/compound in your pipeline of potential candidates, FITC-SA-ZAP can provide a quick, simple and cost effective approach (Estimate 25 µg of FITC-SA-ZAP mixes equimolar with 35 µg of biotinylated antibody). Developing multiple conjugates of antibodies or peptides with saporin and FITC requires time and the expertise to ensure the continued functionality of both individual components after conjugation. With FITC-SA-ZAP, the reaction takes place at room temperature and is complete in 30 minutes (Ancheta, 2023) and is then ready to be diluted and applied to your cells.
Conclusions
- FITC-SA-ZAP can screen antibody and small molecule therapeutic candidates for biomarkers that internalize effectively upon binding.
- Easy-mix protocol for conjugate: reaction takes place at room temperature and is complete in 30 minutes and is then ready to be diluted and applied to your cells
- Can apply to multiple applications without need for multiple conjugates
- Quick turn-around time for data: 1 hr for fluorescence and 72 hr for cell death
- Cost effective: 25 µg of FITC-SA-ZAP mixes equimolar with 35 µg of biotinylated antibody
- Potential for in vivo use for tracking of binding and internalization
References
- Tan HL, Yong C, Tan BZ, Fong WJ, Padmanabhan J, Chin A, Ding V, Lau A, Zheng L, Bi X, Yang Y, Choo A (2018) Conservation of oncofetal antigens on human embryonic stem cells enables discovery of monoclonal antibodies against cancer. Sci Rep 8:11608. doi: 10.1038/s41598-018-30070-z PMID: 30072783
- Yuan X, Yang M, Chen X, Zhang X, Sukhadia S, Musolino N, Bao H, Chen T, Xu C, Wang Q, Santoro S, Ricklin D, Hu J, Lin R, Yang W, Li Z, Qin W, Zhao A (2017) Characterization of the first fully macropinocytosing human antibodies human anti-TEM1 scFv in models of solid tumor imaging and immunotoxin-based therapy. Cancer Immunol Immunother 66:367-378. doi: 10.1007/s00262-016-1937-z PMID: 27933426
- Forsyth PA, Krishna N, Lawn S, Valadez JG, Qu X, Fenstermacher DA, Fournier M, Potthast L, Chinnaiyan P, Gibney GT, Zeinieh M, Barker PA, Carter BD, Cooper MK, Kenchappa RS (2014) p75 neurotrophin receptor cleavage by α- and γ-secretases is required for neurotrophin-mediated proliferation of brain tumor-initiating cells. J Biol Chem 289(12):8067-8085. doi: 10.1074/jbc.M113.513762 PMID: 24519935
- Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 23(10):1985-1996. doi: 10.1158/1055-9965.EPI-14-0275 PMID: 25053711
- Ancheta LR, Shramm PA, Bouajram R, Higgins D, Lappi DA (2023) Streptavidin-saporin: Converting biotinylated materials into targeted toxins. Toxins 15(3):181. doi: 10.3390/toxins15030181

