Selective ablation of IB4+ primary afferent neurons reduces mechanical and cold hyperalgesia in an EAE mouse model of multiple sclerosis
Kayla L. Nguyen1, Sydney R. Lamerand1, Ronit P. Deshpande1, Bradley K. Taylor1,2
1Department of Anesthesiology and Perioperative Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA
2Center for Neuroscience at the University of Pittsburgh, Pittsburgh, PA
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system. Over 50% of patients with MS suffer from neuropathic pain (MSNP).1,2 Patients also show poor response to analgesic drugs, prompting calls for a better understanding of underlying mechanisms.3 A major tool to interrogate such mechanisms are rodent models of experimental autoimmune encephalomyelitis (EAE), which mimic the neuroimmune and somatosensory pathology of MS and MSNP.3,4 While most studies have used EAE models to focus on CNS mechanisms, the contribution of primary afferent neurons (PANs) to MSNP is just now emerging.5-7 For example, EAE sensitizes both small and medium-to-large diameter PANs leading to their hyperexcitability.5 EAE also increases after hyperpolarization in small-diameter PANs leading to an increase in their firing of action potentials.7 We chose to focus on the Isolectin B4 (IB4) subpopulation of small-diameter nonpeptidergic C-nociceptors because they mediate acute mechanical nociception and become hypersensitive after nerve injury.8
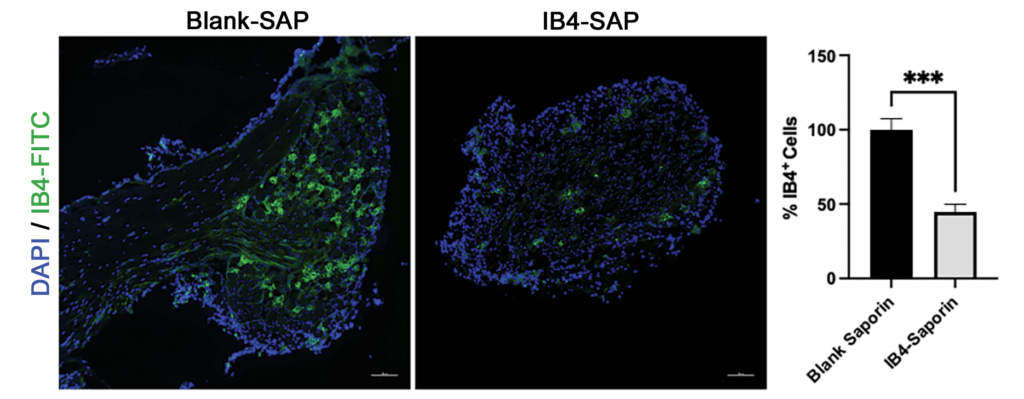
Figure 1: Intrathecal IB4-SAP (1.5 µg) significantly depleted nonpeptidergic C-fibers in lumbar DRG of B6 mice (representative IB4-FITC staining, scale bar = 100 µm; Bar graph represents the mean ± S.E.M. n=4; T-Test; ***p<0.001).
To test the hypothesis that IB4+ neurons in the dorsal route ganglion (DRG) contribute to hyperalgesia in EAE, we selectively ablated them with intrathecal injection (1.5 µg in a total volume of 5 µL) of saporin-conjugated IB4+ (IB4-SAP, Cat. #IT-10). Controls received 0.65 µg of blank saporin (Blank-SAP, Cat #IT-21). Figure 1 illustrates that IB4-SAP reduced IB4 staining by over 50% as compared to controls.
Next, we induced EAE in 10-week-old female mice with two subcutaneous injections of myelin oligodendrocyte glycoprotein (MOG35-55) peptide (100 µg) and/or complete Freund’s adjuvant (CFA) containing 200 µg of heat-inactivated Mycobacterium tuberculosis H37Ra9 (4 mg/ml). As illustrated in Figure 2, EAE mice (but not naïve mice) given CFA or MOG+CFA developed mechanical (von Frey) and cold (acetone) allodynia, but this only persisted in MOG+CFA mice. IB4-SAP was administered 12 days later, and this reduced mechanical and cold hyperalgesia in MOG+CFA mice but had no effect in naïve or CFA-treated controls (Fig. 2).
These data suggest that nonpeptidergic IB4+ C-nociceptors contribute to the maintenance of MSNP. In future studies, we will explore the contribution of nonpeptidergic C-fibers to the induction of MSNP, as well as the contribution of large-diameter A-fibers.
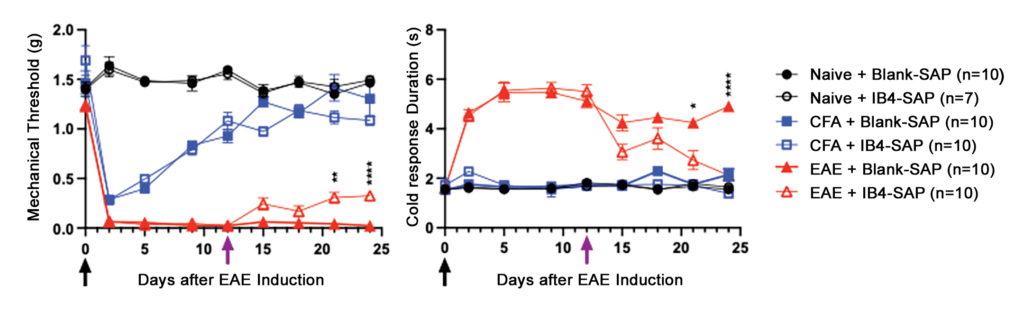
Figure 2: IB4-SAP significantly reduces mechanical (left) and cold (right) hypersensitivity in EAE. Black arrow = 1st EAE immunization, Purple arrow = Blank-SAP/IB4-SAP (Two-way ANOVA; *EAE+saporin vs. EAE+IB4-SAP; Bar represents mean ± S.E.M.)
References
- O’Connor AB, Schwid SR, Herrmann DN, Markman JD, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111.
- Bermejo PE, Oreja-Guevara C, Diez-Tejedor E. [Pain in multiple sclerosis: prevalence, mechanisms, types and treatment]. Rev Neurol. 2010;50(2):101-8.
- Iannitti T, Kerr BJ, Taylor BK. Mechanisms and pharmacology of neuropathic pain in multiple sclerosis. Curr Top Behav Neurosci. 2014;20:75-97.
- Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 2011;164: 1079–1106.
- Yousuf MS, Noh MC, Friedman TN, Zubkow K, Johnson JC, Tenorio G, Kurata HT, Smith PA, Kerr BJ. Sensory neurons of the dorsal root ganglia become hyperexcitable in a T-cell-mediated MOG-EAE model of Multiple Sclerosis. eNeuro. 2019;6(2).
- Khan N, Smith MT. Multiple sclerosis-induced neuropathic pain: pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology. 2014;22(1):1-22.
- Yousuf MS, Samtleben S, Lamothe SM, Friedman TN, Catuneanu A, Thorburn K, Desai M, Tenorio G, Schenk GJ, Ballanyi K, Kurata HT, Simmen T, Kerr BJ. Endoplasmic reticulum stress in the dorsal root ganglia regulates large-conductance potassium channels and contributes to pain in a model of multiple sclerosis. FASEB J. 2020;34(9):12577-12598.
- Wang C, Gu L, Ruan Y, Geng X, Xu M, Yang N, Yu L, Jiang Y, Zhu C, Yang Y, Zhou Y, Guan X, Luo W, Liu Q, Dong X, Yu G, Lan L, Tang Z. Facilitation of MrgprD by TRP-A1 promotes neuropathic pain. FASEB J. 2019;33(1):1360-1373.
- Murphy KL, Fischer R, Swanson KA, Bhatt IJ, Oakley L, Smeyne R, Bracchi-Ricard V, Bethea JR. Synaptic alterations and immune response are sexually dimorphic in a non-pertussis toxin model of experimental autoimmune encephalomyelitis. Exp Neurol. 2020;323:113061.
