Brain imaging techniques that use vascular signals to map changes in neuronal activity rely on the coupling between electrophysiology and hemodynamics, a phenomenon referred to “neurovascular coupling” (NVC). It is unknown whether this relationship remains reliable under altered brain states associated to acetylcholine (ACh) levels, such as attention and arousal, and in pathological conditions like Alzheimer’s disease. Therefore, the authors assessed the effects of varying ACh tone on whisker evoked-NVC responses in rat barrel cortex, measured by cerebral blood flow (CBF) and neurophysiological recordings (local field potentials, LFPs).
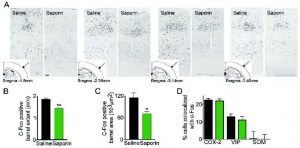
Decreased ACh tone reduces the size and extent of the activated (c-Fos) barrel. Reduced cortical cholinergic innervation with saporin was associated with a significantly reduced whisker stimulation-induced c-Fos upregulation (A) (black nuclei) in the contralateral barrel cortex compared to controls (n=4/group). Scale bar: 100 um. (B) Cholinergic lesion decreased the rostro-caudal extent of the c-Fos-positive cells, and (C) the area they occupied in layer IV of the activated barrel. In control rats, the barrel started on average at bregma level -1.8 mm, while in lesioned animals it started at ~-2.2 mm. In both conditions the c-Fos barrel disappeared at around bregma -3.7 mm. Double immunohistochemistry of c-Fos and specific neuronal markers (D) in the responsive barrel indicated that the typical pattern of activated COX-2 pyramidal cells and VIP interneurons, with virtually no SOM interneurons, was unaltered in ACh-depleted rats as compared to controls (*p<0.05; **p<0.01; ***p<0.001, Student’s t-test).
The authors found that acutely enhanced ACh tone significantly potentiated whisker-evoked CBF responses through muscarinic ACh receptors, and concurrently facilitated neuronal responses illustrated by increases in the amplitude and power in high frequencies of the evoked LFPs. However, the cellular identity of the activated neuronal network within the responsive barrel was unchanged, as characterized by c-Fos upregulation in pyramidal cells and GABA interneurons co-expressing vasoactive intestinal polypeptide. In contrast, chronic ACh deprivation hindered whisker-evoked CBF responses, and the amplitude and power in most frequency bands of the evoked LFPs, and reduced the rostro-caudal extent and area of the activated barrel without altering its identity. ACh depletion was achieved via unilateral icv injection (4 mcg/2 mcl) of the selective cholinotoxin 192 IgG-SAP (Cat# IT-01) or saline (control rats), as described before (Kocharyan et al., 2008).[1] Correlations between LFP power and CBF, used to estimate NVC, were enhanced under high ACh tone and significantly disturbed by ACh depletion. They conclude that ACh is not only a facilitator, but also a prerequisite for the full expression of sensory-evoked NVC responses, indicating that ACh may alter the fidelity of hemodynamic signals in assessing changes in evoked neuronal activity.
Questions? Ask a Product Manager
