by Masahiro Sato1 (Ph. D.) and Satoshi Watanabe2 (Ph. D.)
1Section of Gene Expression Regulation, Frontier Science Research Center, Kagoshima University, Kagoshima 890-8544, Japan:
2Animal Genome Research Unit, Division of Animal Science, National Institute of Agrobiological Sciences, Ibaraki 305-8602, Japan
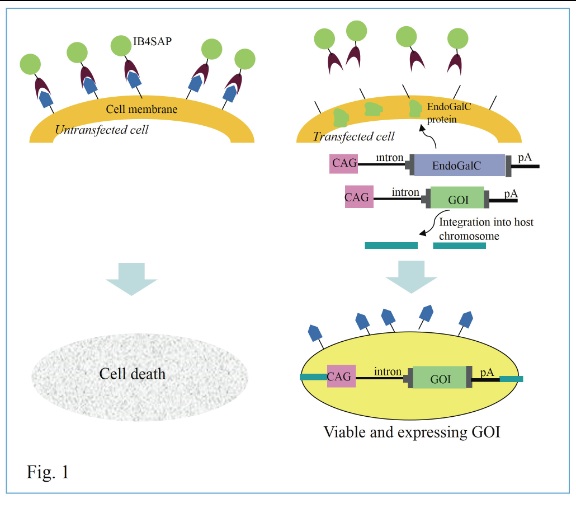
Isolation of stable transfectants is one of the important steps for exploring biological functions of gene of interest. Most studies have employed drug resistance genes, such as the neomycin resistance gene (neo), to eliminate unwanted, untransfected cells after transfection. In such cases, the drug resistance genes are integrated into host chromosomes upon transfection so that they synthesize proteins capable of degrading drugs present in medium. However, this method often causes unwanted byproducts that are occasionally toxic to cells if they are continuously cultivated in the drug-containing medium. Therefore, drug-free selection of stable transfectants is necessary. Previously, we have assessed this problem and have provided a way to obtain transfectants efficiently in the absence of drug selection. Our system is based on co-transfection with a vector carrying a gene of interest (GOI) and a vector (pCAG/EndoGalC) carrying a gene encoding Clostridium perfringens-derived endo-ß-galactosidase C (EndoGalC), which cleaves a specific cell-surface carbohydrate, called the a-Gal epitope, expressed in most mammalian cells, except in humans and Old World monkeys.
Transient expression of EndoGalC should lead to resistance in some transfected cells to isolectin BS-I-B4 conjugated to saporin (rIB4-SAP; #IT-10; Advanced Targeting Systems Inc.), which causes cell death of a-Gal epitope-expressing untransfected cells, mainly because rIB4-SAP, internalized via specific binding to the cell-surface a-Gal epitope, inhibits protein synthesis.[1,2] During the period (~3 days after transfection) of transient expression of exogenous DNA (pCAG/EndoGalC), expression of a-Gal epitope on the cell surface is lost, allowing the cell to survive rIB4-SAP treatment (as schematically depicted in Fig. 1). Concomitantly, the co-introduced plasmid carrying the GOI has a chance to be integrated into host chromosomes. Untransfected cells continue to express the a-Gal epitope, which is specifically recognized by rIB4-SAP, and are eliminated after about 10 days of cultivation (Fig. 1). Thus, the surviving cell population is expected to express the GOI and the a-Gal epitope, since pCAG/EndoGalC introduced into a cell is lost during the 10-day cultivation after rIB4-SAP treatment. This concept has been previously explored by us,[3] in which we demonstrated that the GOI could be effectively integrated into host chromosomes via the piggyBac system after rIB4-SAP treatment.
This drug-free acquisition of stably transfected cells is a very simple and convenient system. We prepared only two vectors, an EndoGalC-expression vector (in circular form) and a vector (in linearized form) carrying a GOI (Fig. 2). These vectors were mixed with trypsinized porcine fetal fibroblasts and were then delivered to the cells via electroporation. Immediately after electroporation, all transfected cells were seeded onto a dish containing normal medium and cultured for 2–3 days. The cells were then trypsinized and treated with 80 µg/mL rIB4-SAP in a 0.5-mL microfuge tube for 2 h at 37°C. Then, these cells were seeded in normal medium and cultured for ~10 days until colonies developed. The colonies were stained with Giemsa and were seen to express the GOI (tdTomato), as shown in the images at the bottom of Fig. 2. This system does not require selective drugs such as G418. Therefore, it does not require a pilot study to test the effectiveness of the drugs using untransfected cells. Furthermore, it will be useful for gene delivery to cells that are resistant to several selective drugs.
|
 |
References: (back to top)
- Akasaka E, Watanabe S, Himaki T, Ohtsuka M, Yoshida M, Miyoshi K, Sato M. (2010) Enrichment of xenograft-competent genetically modified pig cells using a targeted toxin, isolectin BS-I-B4 conjugate. Xenotransplantation 17(1):81-89.
- Sato M, Akasaka E, Saitoh I, Ohtsuka M, Nakamura S, Sakurai T, Watanabe S. (2013) Targeted toxin-based selectable drug-free enrichment of Mammalian cells with high transgene expression. Biology (Basel) 2(1):341-355.
- Sato M, Inada E, Saitoh I, Matsumoto Y, Ohtsuka M, Miura H, Nakamura S, Sakurai T, Watanabe S. (2015) A combination of targeted toxin technology and the piggyBac-mediated gene transfer system enables efficient isolation of stable transfectants in nonhuman mammalian cells. Biotechnol J 10(1):143-153.
