Contributed by Lique M. Coolen, University of Michigan
Introduction from Dr. Lappi: An article by Lique Coolen’s group[5] was the 1000th article in PubMed in response to the search word ‘saporin.’ Dr. Coolen has graciously agreed to describe the latest in her fascinating work.
Male sexual behavior consists of many different aspects, with ejaculation being the most rewarding. Ejaculation is a complex reflex controlled by a central pattern generator in the lumbosacral spinal cord, named the spinal ejaculation generator.[1] This generator receives sensory inputs during mating via the dorsal penile nerve and triggers emission and expulsion of seminal fluids via projections to autonomic and motor neurons in the lumbosacral spinal cord. For a long time it was hypothesized that the integration of sensory inputs and autonomic/motor outflow is mediated by a key group of interneurons located in the lumbar spinal cord. Indeed, 10 years ago, our laboratory identified this key population of inter-neurons as a group of spinothalamic cells located in lumbar levels L3-4 surrounding the central canal in laminae VII and X.[2] Based on their location and axonal projections, this cell group is referred to as lumbar spinothalamic (LSt) cells. These neurons express several neuropeptides, including galanin, and exclusively express neurokinin-1 (NK-1) receptors (Figure 1A), thus allowing us to test questions concerning the functional role of LSt cells using saporin conjugated to SSP (Cat. #IT-11), the substance P analog [Sar9, Met(O2)11], from Advanced Targeting Systems. In our previous paper we demonstrated that cell-specific lesions of the LSt cells, completely eliminated ejaculatory behavior, demonstrating the pivotal role of this neural population for ejaculation as the key component of the spinal ejaculation generator.[2] Moreover, we showed that LST cells have projections to the autonomic preganglionic and motor centers controlling ejaculation and are activated by sensory inputs from the dorsal penile nerve;[3,4] stimulation of this nerve triggers ejaculation in mammals (Figure 2).[5] However, several questions remained to be addressed. The spinal ejaculation generator, and thus the ejaculatory reflex, is influenced by inhibitory and excitatory projections from supraspinal sites.[1] Therefore, in the current study we determined that LSt cells were essential for control of ejaculatory reflexes in the absence of these supraspinal influences, using several different sensory stimulation and pharmacological paradigms in anesthetized male rats with transections of the spinal cord.[5]
Methods: Male rats (anesthetized with a ketamine/xylazine mixture) received a laminectomy to expose the L3-L4 spinal segment and bilateral injections (12 x 1 µL) of the selective neurotoxin saporin conjugated to SSP [SSP-SAP (Cat. #IT-11); 4 ng/µL] into the spinal cord targeting the entire LSt population (Figure 1B,C). We previously demonstrated that this technique results in specific lesions of the LSt cells without loss of other surrounding neurons in the spinal cord, without loss of NK-1 receptor neurons in the dorsal horn, and without changes in thermal pain perception.[2] Control males received saporin conjugated to a nonsense peptide (Blank-SAP, Cat. #IT-21; 3.68 ng/mL) or had misplaced injections of SSP-SAP. Two weeks following lesion surgeries, males were tested for ejaculatory reflexes induced by stimulation of the dorsal penile nerve, stimulation of the urethra, or by treatment with the D3 dopamine receptor agonist 7-hydroxy-2-N,N-dipropylaminotetralin (7-OH-DPAT; 1 mg/kg s.c.). All males were anesthetized with urethane and the spinal cord was completely transected between thoracic levels T6-8. Ejaculatory reflexes were measured by EMG recordings of the striated perineal muscle, the bulbocavernosus muscle (BCM), as rhythmic bursting of this muscle is characteristic of the ejaculatory reflex in mammals.
Specific lesions of LSt cells completely ablated the BCM bursting characteristic of ejaculation upon stimulation of the dorsal penile nerve, urethra, or dopamine D3 receptors in anesthetized, male rats with spinal transections, while BCM bursting was reliably triggered in all control animals (Figure 3). These data extend our previous findings that LSt cells are essential for ejaculatory behavior in mating animals, thus providing further evidence for the pivotal role of LSt cells in control of ejaculation. Moreover, these data demonstrate the LSt cells control ejaculation via projections within the spinal cord, presumably to autonomic and motor neurons, and independent of supraspinal influences.
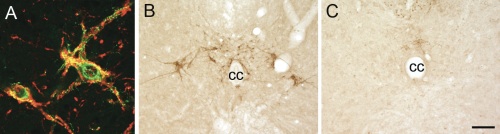 |
Figure 1.A) LSt cells express NK-1 receptors (immunoreactivity for galanin in green and NK-1 in red.[6] Representative images showing presence of galanin-immunoreactive LSt cells surrounding central canal (cc) in control males (B), and absence in LSt-lesioned males (C).Scale bar indicates 20 µm (A) and 100 µm (B,C). |
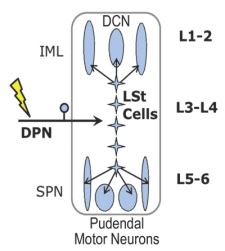 |
Figure 2.Schematic overview of the spinal ejaculation generator. Lumbar spinothalamic (LSt) cells receive sensory inputs via the dorsal penile nerve (DPN) and send axonal projections to preganglionic sympathetic neurons in the central autonomic nucleus (CAN) and intermediolateral cell column (IML), to preganglionic parasympathetic neurons in the sacral parasympathetic nucleus (SPN), and to pudendal motor neurons in the sacral nucleus of the bulbocavernosus (SNB). Spinal lumbar levels are indicated on the right.Modified from Kozyrev et al.[5] |
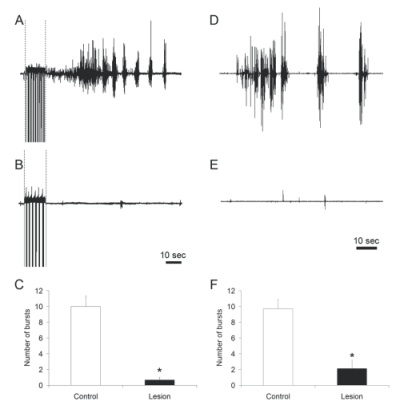 |
Figure 3.Representative examples of bulbocavernosus muscle bursting in control (A, D) and LSt-lesioned males (B, E) following stimulation of the dorsal penile nerve (indicated by dashed lines in A, B) or systemic injections of 7-OH-DPAT (D, E). Quantitative analysis of bulbocavernosus muscle EMG is shown as numbers of bursts (C, F).* indicates significant difference from control animals. |
References: (back to top)
- Coolen, L.M. (2005) Neural control of ejaculation. J Comp Neurol 493:39-45.
- Kozyrev, N., Lehman, M.N. & Coolen, L.M. (2012) Activation of Gastrin-releasing Peptide Receptors in the Lumbosacral Spinal Cord is Required for Ejaculation in Male Rats. J Sex Med. Mar 16. [Epub ahead of print]
- Staudt, M.D., de Oliveira, C.V., Lehman, M.N., McKenna, K.E. & Coolen, L.M. (2010) Activation of MAP kinase in lumbar spinothalamic cells is required for ejaculation. J Sex Med 7:2445-2457.
- Staudt, M.D., de Oliveira, C.V., Lehman, M.N., McKenna, K.E. & Coolen, L.M. (2011) Activation of NMDA receptors in lumbar spinothalamic cells is required for ejaculation. J Sex Med 8:1015-1026.
- Staudt, M. D., Truitt, W. A., McKenna, K. E., de Oliveira, C. V.R., Lehman, M. N. and Coolen, L. M. (2011), A Pivotal Role of Lumbar Spinothalamic Cells in the Regulation of Ejaculation via Intraspinal Connections. J Sex Med. doi: 10.1111/j.1743-6109.2011.02574
- Truitt, W.A. & Coolen, L.M. (2002) Identification of a potential ejaculation generator in the spinal cord. Science 297:1566-1569.
