Contributed by Naomi E. Rance, Melinda. A. Mittelman-Smith and Sally J. Krajewski-Hall
Department of Pathology, University of Arizona College of Medicine
This year, our two posters shared the ATS Poster of the Year Award at the Society for Neuroscience Meeting. These posters documented the use of NK3-SAP, a custom conjugate of saporin (Cat. #IT-63) with a neurokinin 3 receptor (NK3r) agonist, as a tool to ablate NK3r-expressing KNDy neurons in the rat brain. In the first poster, we demonstrated the effectiveness of NK3-SAP for selective ablation of KNDy neurons in the rat arcuate nucleus. In the second poster, this technique was used to examine the role of KNDy neurons in the estrogen regulation of LH secretion and body weight. These data have recently been accepted for publication in Endocrinology.[1]
Although estrogen withdrawal has profound effects on gonadotropin secretion and body weight, the neural circuitry underlying these effects is not well understood. Recent studies have focused on a group of arcuate neurons expressing estrogen receptor alpha and the KNDy peptides in the estrogen modulation of reproduction.[2-3] Because these neurons express NK3r,[4] we hypothesized that they could be ablated using saporin conjugated to a selective NK3r agonist. NK3-SAP was obtained as a custom conjugate from Advanced Targeting Systems. Pilot studies validated the selectivity of NK3-SAP for ablation of NK3r neurons in the rat brain.
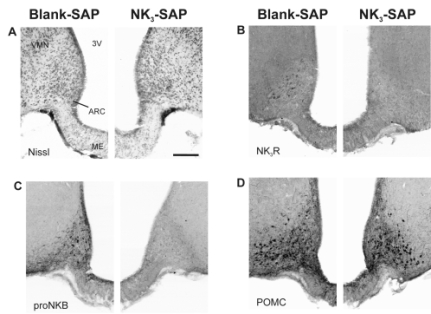
Twenty-four female rats were ovariectomized and received bilateral arcuate microinjections of either NK3-SAP or Blank-SAP (control, Cat. #IT-21). Three weeks later, animals were implanted with silastic capsules containing 17β-estradiol (E2) and 11 days after that, they were sacrificed. Body weights and blood samples were taken at the beginning of the experiment, three weeks after ovariectomy, and at 11 days after E2 -treatment. Immunohistochemical studies revealed ablation of KNDy neurons by NK3-SAP. There was near-complete loss of NK3r neurons in the arcuate nucleus with a 94-98% reduction in the number of kisspeptin and neurokinin B neurons, compared to Blank-SAP rats (Figure 1). In contrast, proopiomelanocortin, neuropeptide Y and GnRH immunoreactive elements were preserved.
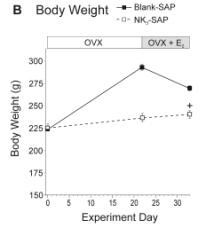
We found that ablation of KNDy neurons resulted in marked changes in LH secretion and the E2 modulation of body weight. In control animals, ovariectomy markedly increased serum LH and body weight and these effects were reversed by replacement with E2. In contrast, NK3-SAP-injected rats did not exhibit a significant rise in serum LH in response to ovariectomy, and serum LH was lower in these animals regardless of estrogen status (Figure 2A). Surprisingly, the effects of ovariectomy and E2 on body weight were blocked in rats with KNDy neuron ablation (Figure 2B). These data show an essential role of arcuate KNDy neurons for tonic LH secretion, the rise in LH in response to ovariectomy, and the E2 modulation of body weight.
References: (back to top)
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. (2012) Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology April 16 Epub ahead of print.
- Rance NE. (2009) Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111-122.
- Lehman MN, Coolen LM, Goodman RL. (2010) Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arucate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479-3489.
- Burke MC, Letts PA, Krajewski SJ, Rance NE. (2006) Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712 -726.