Contributed by Megan St. Peters and Martin Sarter
Departments of Psychology at Ferrum College, Ferrum, VA and University of Michigan, Ann Arbor, MI
Increasing attentional “effort” as a result of challenging circumstances, and as a function to maintain or recover attentional performance, is pervasive in our daily lives.[1] Attentional effort is more than a function of task difficulty; it is also a function of the subject’s motivation to perform. Consider, for instance, your attention focused on driving when you are alone on a well-travelled road with no traffic around, in contrast to when a police vehicle is behind you. Indeed, in many real-world scenarios, attention and motivation are interwoven.
Motivational-incentive processing involves mesolimbic circuitry, particularly the dopaminergic midbrain and the nucleus accumbens (NAc).[2,3] Top-down control of attention relies on frontoparietal cortical regions.[4,5] However, the precise circuitry underlying motivation’s modulation of attention had remained largely undefined.
Here, we show that interactions between the NAc and the basal forebrain corticopetal cholinergic projection system are essential components of the circuitry involved in the motivated recruitment of attention. We do this by using the operant sustained attention task (SAT). This task requires animals to press a lever to indicate the presence (signal trials) or absence (nonsignal trials) of a cue light. Correct responses to signal and nonsignal trials (“hits” and “correct rejections,” respectively) result in a water reward. Incorrect responses (“misses” and “false alarms,” respectively), are not rewarded. Animals undergo daily 40-minute sessions, which are divided into five 8-minute blocks for analyses. Once trained to reach criterion (70% correctly identified signal and non-signal trials) animals are also exposed to the more challenging distractor version of the task (dSAT). Overall attentional performance was determined using SAT/dSAT scores, that are derived from performance in both signal and non-signal trials. This measure ranges from -1.0 to +1.0, with -1.0 indicating that all responses were misses and false alarms, 0 indicating an inability to discriminate between signal and nonsignal events, and +1.0 indicating that all responses were hits and correct rejections. For a more comprehensive description of the methods and results, please see reference 6.
In this first set of studies, we examined whether stimulation of NAc benefits attentional performance during the SAT and dSAT. After animals were trained to criterion, they were implanted with bilateral guide cannulas in either the shell or the core region of the NAc. After surgery, animals were habituated to performing the task while being tethered. Each animal received four infusions – two during SAT and two during dSAT. For each condition, the animal received an infusion of vehicle (saline; 0.9%) or NMDA (Sigma-Aldrich), dissolved in 0.9% saline, so that each animal served as its own control. Animals received 0.5 µL of drug or vehicle into each site simultaneously, using a microinfusion pump (Model CMA/100; Carnegie Medicine) at the rate of 0.5 µL/minute. Infusions occurred during the first two minutes of block 2 to enable demonstration of similar SAT performance pre-infusion.
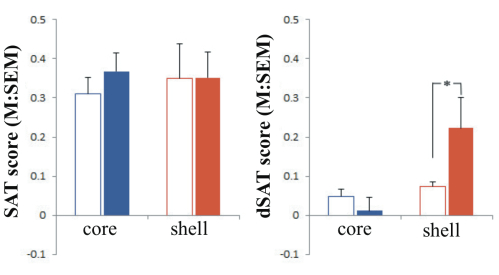
Effects of NAc shell and core infusions on SAT and dSAT performance (omnibus test). The analysis of the effects of group (shell vs. core), task type (SAT vs. dSAT), block of trials (t1–t5), signal duration (500, 50, 25 ms), and dose of NMDA (0, 0.067, 0.33, 1.01 nmol/hemisphere) indicated a significant interaction between all factors (F(8,464) = 2.72, p= 0.01). No differences were found between conditions pre-infusion (data shown in reference 6). To be concise, the most effective dosage found for infusions are reported in this summary. Post hoc analyses revealed that stimulation of NMDA receptors in the shell or core had no effect during SAT performance. When attentional demands were increased by the dSAT, stimulation of NMDA receptors (0.33 nmol) in the shell enhanced performance (see Figure 1). NMDA stimulation continued to have no effect when infused in the core during dSAT.
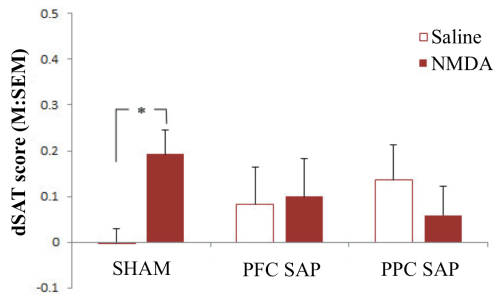
In the second set of studies, we wanted to see if the benefits afforded by stimulation of the NMDA receptors in the shell were related to its neural connectivity with the cortical cholinergic system. Animals were randomly assigned to receive sham surgeries, cholinergic deafferentation of the prefrontal cortex (PFC), or cholinergic deafferentation of the posterior parietal cortex (PPC). Sham surgeries were performed using the control immunotoxin, mouse IgG-SAP (Cat. #IT-18), whereas cholinergic deafferentation was produced using 192 IgG-SAP (Cat. #IT-01). All animals that received these surgeries (sham, PFC, or PPC) also received bilateral implantation of guide cannulas in the shell region of the NAc. They were tested using identical parameters to the previous study, receiving saline and NMDA infusions. As shown in Figure 2, the infusions in the sham animals replicated the results in Experiment 1 – namely, infusions of NMDA enhanced performance during dSAT (see left panel, Figure 2). The benefits of NMDA during dSAT were not observed in the PFC or PPC deafferented animals (middle and right panels, Figure 2).
These results demonstrate that motivation’s modulation of attention is reliant on the interactions between the shell of the NAc and the basal forebrain corticopetal cholinergic projection system. Interestingly, this interaction is observed only when attentional systems are taxed during dSAT, as the benefits of NMDA infusions were not observed during SAT, suggesting this circuitry specifically underlies the motivated recruitment of the basal forebrain corticopetal cholinergic projection system. Further, stimulation of the NAc core offered no benefits to attentional performance, showing the selectivity of the neural circuitry. Our results define a mesolimbic-basal forebrain cortical system that mediates the motivated activation of attentional mechanisms. Strategies designed to treat attentional impairments or enhance attentional performance may benefit from adopting broader concepts that integrate motivational-attentional interactions and from exploiting the multiple targets known to influence mesolimbic-basal forebrain circuitry.
References: (back to top)
- Sarter M, Gehring WJ, Kozak R (2006) More attention must be paid: the neurobiology of attentional effort. Brain Res Rev 51:145–160.
- Knutson B et al. (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159.
- Adcock RA et al. (2006) Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50:507–517.
- Wager TD, Jonides J, Reading S (2004) Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage 22:1679 –1693.
- Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H (2010) Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage 49:3426 –3435.
- St. Peters M, Demeter E, Lustig C, Bruno JP, & Sarter M (2011) Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci 31: 9760-71.