Contributed by Tamara King and Frank Porreca Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ, USA
A common symptom of patients with neuropathic pain is spontaneous pain, often described as burning. Some patients also suffer from pain elicited by normally non-noxious stimuli, such as touch, referred to as allodynia. Preclinical studies have relied on enhanced withdrawal responses to normally innocuous tactile stimuli or noxious thermal stimulation. As such, we have learned much about mechanisms driving gain of function responses to evoked stimuli. Indeed, such measures are used as the main translation feature of experimental neuropathic pain models. An important criticism directed against animal models of pain is that behavioral assessment depends on reflexive evoked responses to innocuous or noxious external stimuli. However, the primary complaint from pain patients is that of ongoing pain, i.e., pain that is independent of an external stimulus. As such, the reliance of testing protocols on evoking a nocifensive response has been considered a significant barrier for development of new therapies for pain treatment.
We have recently developed an approach that is based on the knowledge that relief of pain is rewarding in humans. We hypothesized that chronic pain produces an aversive state providing persistent and strong behavioral motivation to seek relief that is rewarding. Reward achieved from removal of an aversive stimulus is denoted as “negative reinforcement” and is applicable to alleviation of the aversive state induced by chronic pain. We explored this concept using the well-characterized conditioned place preference (CPP) assay, in which pairing pain relief with a distinct context was demonstrated to result in increased time spent in that context.[1] Importantly, CPP to a context paired with pain relief was only observed in nerve-injured rats, leading to the “unmasking” of spontaneous experimental neuropathic pain.[1-3] Validation of this model for detecting ongoing, or non-evoked, pain, was performed with rats across different models of experimental nerve injury (i.e., spinal nerve ligation or spared nerve injury) and treatments (e.g. spinal clonidine, ω-conotoxin) known to alleviate spontaneous neuropathic pain (pain at rest) in clinical reports.[1] This approach allows for investigation of mechanisms that may promote pain as well as those that may be associated with pain relief. For example, this approach demonstrated that blocking descending pain facilitatory pathways from the RVM produces robust CPP, indicating that descending pain facilitation from the RVM promotes nerve-injury induced spontaneous pain.[1-3]
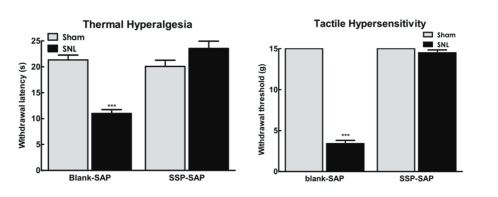
With support provided by the National Institutes of Health, the role of TRPV1-positive sensory fibers and spinal NK-1 positive cells in driving the evoked and spontaneous components of neuropathic pain were determined.[2] At the level of the spinal dorsal horn, ablation of NK-1 receptor-expressing cells with the substituted SP analog, Sar9Met(O2)11 substance P attached to saporin (SSP-SAP; Cat. #IT-11), blocked nerve-injury thermal and tactile hypersensitivity as well as ongoing pain. Immunhistochemical analysis of spinal NK-1 receptor expression following behavioral testing verified elimination of NK-1 receptors within the spinal dorsal horn at time of behavioral testing (Fig 1).
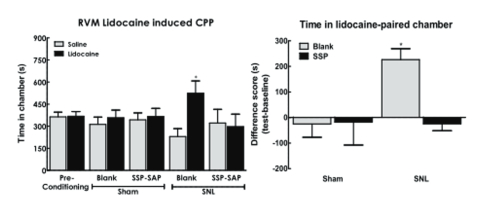
Baseline thermal and tactile sensory thresholds were determined followed by spinal nerve ligation (SNL) or sham (control surgeries). Analysis of sensory thresholds 14 days following surgery by an experimenter blinded to the treatment conditions demonstrated that rats that received blank-SAP (control; Cat. #IT-21) developed robust thermal and tactile hypersensitivity whereas rats that had been treated with SSP-SAP showed normal thermal and tactile sensory thresholds, equivalent to sham- treated rats (Fig 2A,B respectively). SNL-induced spontaneous pain was assessed using CPP to a context paired with pain relief induced by RVM lidocaine. Rats had RVM cannulas implanted followed by a 1-week recovery period followed by SNL or sham (control surgeries). Rats were placed in 3 chamber conditioning boxes with 2 pairing chambers distinguished by texture (rough vs. smooth floors) and visual cues (striped vs. solid gray walls). Preconditioning analysis of time spent in the conditioning chambers demonstrated no difference in time spent in the chambers, indicating no pre-conditioning chamber bias (Fig 3A). Conditioning day occurred 14 days post-SNL or sham surgery. Rats received RVM saline and were immediately confined to one of the pairing chambers for 30 min. Four hours later, rats received RVM lidocaine and were immediately confined to the opposite pairing chamber for 30 min. RVM lidocaine selectively induced CPP in SNL rats treated with blank-SAP (Fig 3A,B). In contrast, SNL rats that had been treated with SSP-SAP spent equivalent time in the RVM saline and lidocaine paired chambers (Fig 3A), indicating no increase in time spent in the RVM lidocaine paired chamber (Fig 3B). These findings indicate that elimination of the NK-1 receptor-expressing cells blocks development of nerve-injury induced spontaneous pain.
These data suggest that spontaneous neuropathic pain likely depends on spinal NK-1 positive ascending projections offering opportunities for exploration of therapeutic interventions.
References: (back to top)
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci 2009; 12(11):1364-1366.
- King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain 2011.
- Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy Pain 2011; in press.