Contributed by Lisa E. Goehler and Ronald P.A. Gaykema
Center for the Study of Complementary and Alternative Therapies, University of Virginia School of Nursing, Charlottesville, VA 22908
Fatigue, experienced as reduced motivation and motor activity, is common in acute and chronic disease, including heart disease, cancer and cancer chemotherapy. Fatigue is part of a constellation of symptoms referred to as “sickness behavior,” which is mediated by the brain and results from physiological and psychological challenges, including inflammation and stress.[1,9] It contributes to poor quality of life, but as yet there are no effective treatments.[9,10] A major hurdle to developing effective treatments for fatigue is the lack of knowledge regarding the mediating brain mechanisms that produce the symptoms. However, we recently determined that catecholamine neurons provide a connection between brain regions that respond directly to signals generated in the body (such as inflammation) and other regions involved in modulating brain activity related to behavior, that likely contribute to the neurocircuitry of fatigue.[2] We were able to make this determination by using targeted lesions of catecholamine neurons with saporin-conjugated anti- dopamine beta hydroxylase (anti-DBH-SAP; Cat. #IT-03).
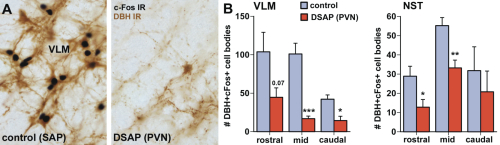
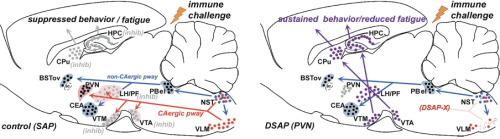
Our previous studies [3-8] indicated that suppression of activity of brain regions in the hypothalamus and midbrain that modulate behavioral arousal contributes to fatigue symptoms associated with immune challenge that induces sickness. These brain regions receive input from catecholamine neurons, located in the nucleus of the solitary tract (NST) and ventrolateral medulla (VLM) of the caudal brainstem, that respond to stress and inflammation (Fig. 1A), suggesting that these neurons may serve as a link between physiological challenge and fatigue.
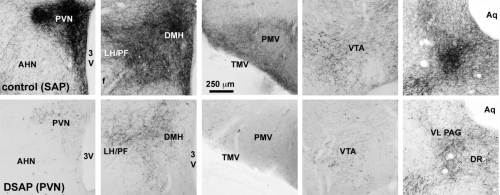
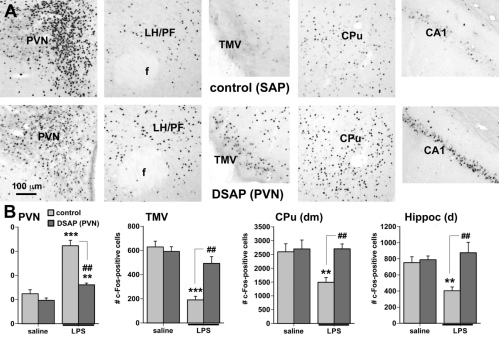
To determine whether catecholaminergic neurons in the caudal brainstem contribute to symptoms of fatigue, we assessed exploratory motor behavior [11] in immune-challenged animals that had previously received injection of anti-DBH-SAP into the paraventricular nucleus of the hypothalamus. This region receives extensive input from the catecholamine neurons of the NST and VLM, and pilot studies showed that injections dramatically reduce numbers of these neurons from caudal brainstem sources (Fig. 1B), but have little effect on other neurons such as those in the locus coeruleus. Control animals were injected with Mouse IgG-SAP (Cat. #IT-18). To explore brain regions potentially influenced in the context of fatigue and by activity of the caudal brainstem catecholaminergic neurons, we assessed two contributing factors. 1) The loss of DBH-positive neurons and innervation (Fig. 2), and 2) The brain activation patterns (reflected by the induction of the cell activation marker c-Fos) normally evident during exploratory behavior during immune challenge, and how the lesion modifies the pattern (Fig. 3A).
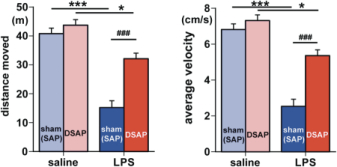
Exploratory behavior was associated with activation in brain areas involved in behavior and motivation, including the hippocampus, dorsal striatum, and ventral tegmental area, and in hypothalamic neurons involved in arousal. Activation of these areas was greatly reduced after immune challenge, but targeted lesion of catecholaminergic projections with anti-DBH-SAP prevented reductions in exploratory behavior (Fig. 4), and completely prevented the suppressive effects of immune challenge on c-Fos expression (Fig. 3B). The findings support the idea that functional inhibition of neuronal populations associated with exploratory behavior, including the ventral tegmental area, dorsal striatum, hippocampus and the hypothalamus could contribute to the behavioral deficits occurring during illness due to inflammatory challenge. Caudal medullary catecholamine projections originating in the VLM and NST provide a link between immune-responsive brainstem regions and forebrain areas that control behavior (Fig. 5).
Selected References: (back to top)
- Dantzer, R., Kelley, K.W., 2007. Brain Behav Immun 21, 153.
- Gaykema, R.P.A., Goehler, L.E. 2010. Brain Behav Immun doi:10.1016/j.bbi.2010.11.005
- Gaykema, R.P.A., Goehler, L.E., 2009. Brain Behav Immun 23, 926.
- Gaykema, R.P.A., Chen, C.-C, Goehler, L.E., 2007. Brain Res 1130, 130.
- Gaykema, R.P.A., Park, S.-M., McKibbin, C.R., Goehler, L.E., 2008. Neurosci 152, 273.
- Gaykema, R.P.A., Daniels, T.E., Shapiro, N.J.,.Thacker, G.C., Park, S.-M., Goehler, L.E. 2009. Brain Res, 1294, 61.
- Goehler, L.E., Gaykema, R.P.A., Opitz, N., Reddaway, R., Badr, N.A., Lyte, M., 2005. Brain Behav Immun 19, 334.
- Marvel, F.A., Chen, C.-C., Badr, N. A., Gaykema, R. P. A., Goehler, L. E., 2004. Brain Behav Immun 18, 123.
- Miller, A.H., 2009. Brain Behav Immun 23, 149.
- Musselman, D.L., Somerset, W.I., Guo, Y., Manatunga, A.K., Porter, M., Penna, S., Lewison, B., Goodkin, R., Lawson, K., Lawson, D., Evans, D.L., Nemeroff, C.B., 2006. J Clin Psych 67, 288.
- Stone, E.A., Lehmann, M.L., Lin, Y., Quartermain, D., 2006. Biol Psych 60:803.