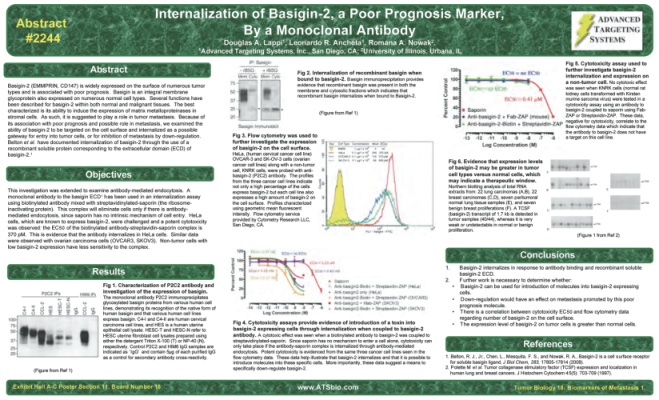
At this year’s AACR meeting, Leonardo Ancheta, scientist and Product Manager from ATS, presented a poster on our new monoclonal antibody to Basigin-2 (Cat. #AB-42). The poster describes our work with the antibody and the immunotoxin made from it (Cat. #IT-54). Basigin-2 (EMMPRIN, CD147) is widely expressed on the surface of numerous tumor types and is associated with poor prognosis. Because of its association with poor prognosis and possible role in metastasis, we examined the ability of basigin-2 to be targeted on the cell surface and internalized as a possible gateway for entry into tumor cells, or for inhibition of metastasis by down-regulation.
Our study reached the following conclusions: 1) Basigin-2 internalizes in response to antibody binding and recombinant soluble basigin-2 ECD. 2) Further work is necessary to determine whether a) Basigin-2 can be used for introduction of molecules into basigin-2 expressing cells, b) Down-regulation would have an effect on metastasis promoted by this poor prognosis molecule, c) There is a correlation between cytotoxicity EC50 and flow cytometry data regarding number of basigin-2 on the cell surface, and d) The expression level of basigin-2 on tumor cells is greater than normal cells.
ATS will be continuing its research with basigin-2 with a SBIR Phase I grant from the National Cancer Institute. The purpose of this Phase I proposal is to provide strong evidence that Anti-Basigin2-SAP is able to eliminate tumor cells that are highly expressing basigin-2. This will provide a rationale to perform in vivo work in mouse tumor models in Phase II to determine anti-tumor activity and to begin design of the molecule as a meaningful systemic drug for treatment of tumors with high metastatic potential.