Contributed by Arshad M. Khan, Kimberly L. Rapp, Todd A. Ponzio, Graciela Sanchez-Watts and Alan G. Watts; Dept. of Biological Sciences and Neuroscience Research Institute, University of Southern California, Los Angeles, CA; and Laboratory for Neurochemistry, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD
The brain has evolved adaptive mechanisms for coping with stress and responds to stressors in highly stereotyped ways. One of the major physiological responses to stressful stimuli, the secretion of pituitary and adrenal hormones, is controlled by corticotropin-releasing hormone (CRH)-expressing neurons located in the paraventricular nucleus of the hypothalamus (PVH). CRH neuroendocrine neurons constitute the primary control center in the brain for initiating hormonal responses to stress, and the control of these neurons by other parts of the brain has been the subject of intensive investigation. One of the most massive sources of input to these neurons is the collection of axonal inputs originating from subpopulations of catecholaminergic (CA) neurons located in the hindbrain. These CA neurons are critical regulators of the mammalian stress axis, releasing the neurotransmitters epinephrine, norepinephrine and other co-localized peptide hormones (such as neuropeptide Y) onto CRH neuroendocrine neurons.
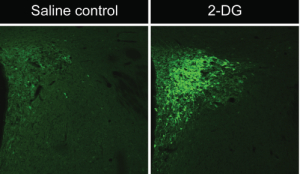
A key question about this input system concerns its role in carrying information to the CRH neuroendocrine system to mediate responses to various stressors. We have previously shown that glycemic challenges such as 2-deoxyglucose (2-DG) or insulin, both of which produce changes in glucose concentrations when injected intravenously, trigger activation of CRH neuroendocrine neurons and are also associated with increases in the plasma concentrations of stress hormones.[1,2] The activation of CRH neuroendocrine neurons under these conditions is deduced, in part, by observing changes in the expression of various cellular markers within these neurons in control vs. stress conditions in laboratory rats. For example, we have shown that systemic insulin or 2-DG injection rapidly elevates levels of the phosphorylated forms of MAP kinases ERK1 and/or ERK2.[1,2] The phospho-ERK1/2 staining appears to be induced selectively by these challenges, as little to no phospho- ERK1/2 appears in the CRH neuroendocrine neurons under basal conditions (Fig. 1).
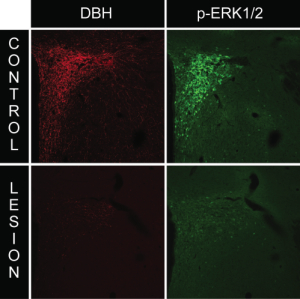
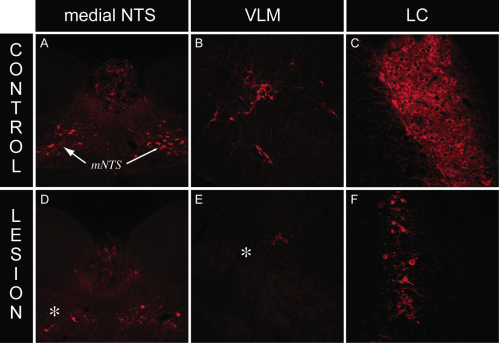
We employed the use of anti-DBH-SAP (Cat. #IT-03) injections to determine whether this phospho-ERK1/2 response requires intact catecholaminergic afferents originating in the hindbrain. Rats given PVH microinjections of anti-dopamine-beta-hydroxylase-saporin conjugate (anti-DBH-SAP) or mouse IgG-saporin control conjugate (Cat. #IT-18) received either normal 0.9% saline vehicle or insulin (2 U/kg, i.v.) and received lethal doses of intravenous anesthesia 30 min later. Brains were processed for DBH and phospho-ERK1/2 immunocytochemistry. Relative to rats receiving sham lesions (injections of control conjugate), anti-DBH-SAP-treated animals displayed a marked loss of DBH immunostaining, indicative of a pronounced loss of catecholaminergic fibers (Fig. 2). This loss was accompanied by loss of the phospho-ERK1/2 response to insulin or 2-DG within these neurons, as evident from the marked reductions of phospho-ERK1/2 immunostaining observed in these animals (Fig. 2). Additionally, hindbrain cell groups in lesioned rats displayed frank loss of DBH staining, indicative of true cell loss in these hindbrain groups as a result of the saporin lesions (Fig. 3).
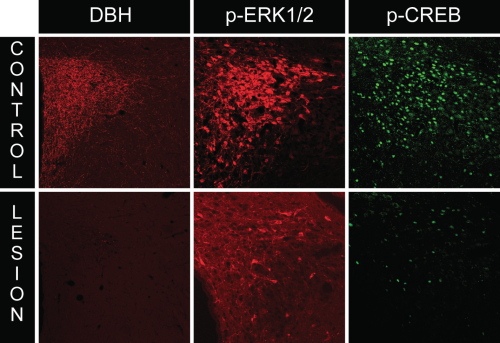
A characteristic response of CRH neuroendocrine neurons to various homeostatic challenges is the initiation of CRH gene transcription, which is thought to be controlled, in part, by the activation (phosphorylation) of the transcription factor, CREB (cyclic AMP response element binding protein). Indeed, we observed phospho-CREB levels to increase markedly in response to insulin and 2-DG in PVH neurons. To ascertain what happens to CREB activation in CRH neuroendocrine neurons in rats receiving anti-DBH-SAP lesions, we performed dual immunocytochemistry for both markers in lesioned rats. As shown in Fig. 4, lesioned animals displayed marked reductions in phospho-CREB, in line with the reductions observed in phospho-ERK1/2 levels.
Collectively, our data demonstrate that rats mount CRH neuroendocrine responses to glycemic challenges in a manner that requires intact catecholaminergic afferents from the hindbrain. Both phospho-ERK1/2 and phospho-CREB signals appear to rely on signals propagating along these catecholaminergic pathways. The targeting of this afferent system using saporin-based immunotoxin conjugates has proven to be a valuable technique for probing the afferent circuitry of this important physiological system.
References: (back to top)
- Khan AM, Watts AG. 2004. Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen- activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology 145(1):351-359.
- Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG. 2007. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: A proposed role during glycemic challenges. The Journal of Neuroscience 27(27):7344-7360.
