Contributed by Kevin Pang and Heidi Smith, Bowling Green State University, Dept Psychology, Bowling Green, Ohio
Hypocretin (or orexin) neurons are located in the hypothalamus and have been implicated in various functions including sleep, arousal and feeding. One area receiving dense hypocretin projections and expressing hypocretin-2 receptors (H-2r) is the medial septum (MS), an area important in learning and memory. In the medial septum, hypocretin-2 receptors are located on cholinergic and GABAergic projection neurons that innervate the hippocampus and other limbic structures. Previous studies demonstrated that activation of H-2r excites both populations of MS neurons. Our studies investigated whether MS neurons that receive hypocretin innervation are important for spatial memory [3].
Male Long Evans rats were administered orexin-saporin (orexin-SAP, Cat. #IT-20) into the MS (0.6 μl) and diagonal band of Broca (DB; 0.4 μl into each DB) at three concentrations: 100, 200 and 300 ng/μl. Following a 2-week recovery period, rats were trained on a water maze task. Each daily session consisted of 6 trials. After behavioral testing, brain sections were stained to visualize cholinergic and GABAergic septohippocampal (SH) neurons using immunocytochemistry for choline acetyltransferase (ChAT) and parvalbumin (PV), respectively.
The damage caused by orexin-SAP is shown in Figure 1. Orexin-SAP at 100 ng/μl primarily damaged GABAergic SH neurons, sparing most cholinergic SH neurons. The concentration of 200 ng/μl caused more neuronal damage. At this concentration, a more substantial loss of cholinergic neurons was observed, but GABAergic neurons again showed more damage than cholinergic neurons.
Finally, the highest concentration of orexin-SAP (300 ng/μl) produced extensive damage to the medial septum, causing shrinkage and non-specific damage of the MS-DB tissue and enlargement of the ventricles. Because of the non-specific tissue damage produced by administration of the highest concentration, we restricted our analysis of the behavioral effects of orexin-SAP to the two lower concentrations.
Rats treated with either 100 or 200 ng/μl were impaired at learning the location of the platform in the water maze (Figure 2). Although mean escape latencies decreased across days for all groups, the rate of learning was slower for rats treated with orexin-SAP as compared to control rats (Figure 2A). Analysis of the first trial of each session was used to assess effects on long-term memory (i.e., 24-hr retention). Rats treated with either dose of orexin-SAP demonstrated a pronounced impairment of long-term memory (Figure 2B). Performance on probe trial for day 4 confirmed the impairments observed on platform trials, but performance on probe trial for day 11 was not different between groups.
In summary, orexin-SAP administered into the MS-DB resulted in a dose-dependent loss of GABAergic and cholinergic SH neurons with a greater damage occurring to GABAergic neurons than cholinergic neurons. Rats treated with 100 and 200 ng/μl of orexin-SAP were impaired in learning the water maze task with pronounced impairment of long-term retention. These results contrast with our previous studies using intraseptal kainic acid administration, which does not impair performance on the water maze or radial maze. The different behavioral results are especially interesting when one considers that 100 ng/μl orexin-SAP preferentially damages GABAergic SH neurons, similar to the damage produced by kainic acid. So far, the effects of these compounds have only been assessed on cholinergic and GABAergic SH neurons. Current studies are attempting to identify the effects of orexin-SAP and kainic acid on non-cholinergic, non-GABAergic MS-DB neurons. Identifying neuronal populations that are differentially affected by these two toxins will help to highlight other MS-DB neurons that may be involved in learning and memory.
In conclusion, these studies demonstrate that orexin afferents to the MS-DB may play a role in modulating memory processes. The results, together with those from previous studies, also suggest an important role of noncholinergic, non-GABAergic MS-DB neurons in spatial memory.
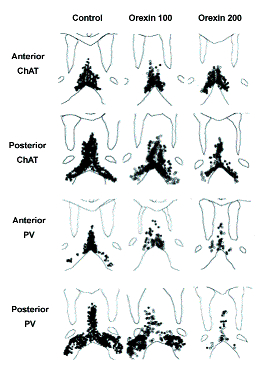
Originally published in Smith & Pang, 2005.
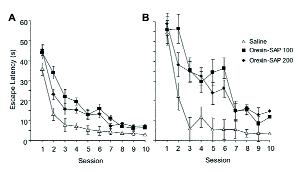
Mean escape latency for daily sessions are shown in Figure 2A. Orexin-SAP significantly impaired the acquisition of a water maze task. No difference was found between the two groups receiving orexin-SAP.
Mean escape latency for the first trial of each daily water maze session is shown in Figure 2B. Although no difference was observed on the first trial of the first session, rats treated with orexin-SAP took significantly longer than the saline-treated group to reach the hidden platform on the first trial of subsequent sessions. No difference was observed between rats treated with the two concentrations of orexin-SAP.
References: (back to top)
- Gerashchenko et al. (2001) Brain Res 913:106-155.
- Pang et al. (2001) Hippocampus 11:814-827.
- Smith, HR and Pang KCH (2005) Neuroscience 132:261-271.
- Wu et al. (2002) J Neurosci 22(17):7754-7765.
