Chronic pain conditions are often caused by ongoing disease states or tissue damage that create sensitization of both the primary afferents (nerves that convey impulses from the outer part of the body) and spinal cord neurons. This sensitization results in a persistent increased sensitivity to both noxious (hyperalgesia) and non-noxious (allodynia) stimuli. Many persistent pain states have proven difficult to treat using currently available pharmacological or surgical approaches that may cause significant unwanted side effects, and often result in significant reduction in quality of life.
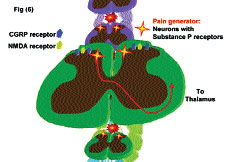
Location and Function of Substance P receptor (SPR)-positive neurons in the spinal cord. SP-SAP specifically targets and eliminates cells that express SPR. Intrathecal administration in the spinal cord kills these spinothalamic neurons that make up less than 5% of the neurons in the spinal cord. These SPR-positive neurons also express CGRP (calcitonin gene-related peptide) and NMDA (N-methyl-D-aspartate or glutamate) receptors. These SPR-positive neurons are labeled “Pain Generators” and it has been shown that their elimination greatly reduces the perception of chronic pain in animal models.
Advanced Targeting Systems is continuing to develop Substance P-Saporin (SP-SAP) as a chronic pain therapeutic. The first of two toxicology studies has begun and we expect to have results from that study in early 2005. All of the preclinical data and scientific publications support the idea that SP-SAP eliminates the chronic pain signal. It accomplishes this by removing a small subset of neurons in the spinal cord.
One patient population that should be targeted is terminal cancer patients who are unresponsive to opioid treatment. These patients have run out of options. The surgical and chemical interventions that are often made by physicians destroy more neurons in a less-specific manner and can even cause a central pain state. We believe SP-SAP can greatly reduce the perception of chronic pain in patients with minimal or no side effects. We believe this because we have seen the effects in two animal models in preclinical studies. Once we have toxicology results the FDA accepts, clinical trials in humans can begin.