Contributed by Jean-Christophe Cassel, PhD, Laboratoire de Neurosciences Cognitives et Comportementales, UMR 7521 Université Louis Pasteur/CNRS, Strasbourg, France & Institut für Experimentelle und Klinische Pharmakologie und Toxikologie der Universität Freiburg, Neuropharmakologisches Labor, Freiburg, Germany. Dr. Cassel and colleagues are the authors of four publications released this quarter using 192-Saporin (Cat. #IT-01)
Trying to understand how various neuroanatomically- and/or neurochemically- defined systems of the brain contribute to elaborate functional outputs at an integrated level –including behavior– roughly relies on two types of approaches. The first consists in exploring brain regions while functional outputs are prepared or expressed (e.g. EEG). The second approach uses more invasive tools (e.g. lesions), alters brain structures, pathways or transmitter systems, and measures functional consequences. One problem with the second approach concerns the neuroanatomical and/or neurochemical selectivity. The more selective the lesion of a particular system in the brain, the more a functional alteration can be linked to the operations enabled by the system. 192 IgG- Saporin has emerged as the most selective toxin to lesion the cholinergic neurons of the basal forebrain. As such, this immunotoxin enables exploration of the contribution to cognitive functions of the septohippocampal and basalocortical cholinergic projection systems while considering the “cholinergic hypothesis of geriatric dysfunctions” proposed by Bartus in 1982.1 From the first behavioral studies with 192 IgG-Saporin lesions, it became evident that cognitive deficits were not as large as expected from previous studies relying on less selective lesions: whether administered intracerebroventricularly (i.c.v.) or intraparenchymally, extremely large cholinergic lesions seemed required to induce clear-cut, though submaximal deficits. There are certainly several ways to look on such data,2 and at least one question worth mentioning: wouldn’t also other neurotransmitter systems participate in those behavioral capabilities commonly attributed to cholinergic functions?3 This question led us to carry out the first experiments in which 192 IgG-Saporin lesions of basal forebrain cholinergic neurons were combined to 5,7-DHT lesions of the ascending serotonergic projection system. In this study, the toxins were injected, separately or in combination, in the ventricles of female rats.4 While none of the single lesions induced any clear-cut behavioral deficit, the combination of both toxins induced a marked alteration of spatial working memory in a radial maze. Part of these results were recently confirmed in male rats.5 In another study,6 the immunotoxin was injected into the septal region and 5,7-DHT into the fimbria-fornix/cingular bundle pathways to achieve neurochemical and neuroanatomical selectivity at once. The cholinergic lesion was large enough to induce a severe deficit in a radial-maze working memory task. Interestingly, damage to the serotonergic innervation of the hippocampus, which had no proper effect, reduced the saporin-induced deficit, suggesting that cholinergic and serotonergic mechanisms interactively contribute to the control of spatial working memory capabilities. These findings also consolidated the idea that reducing the serotonergic tone in the hippocampus may attenuate the deficits produced by a cholinergic denervation of this structure.
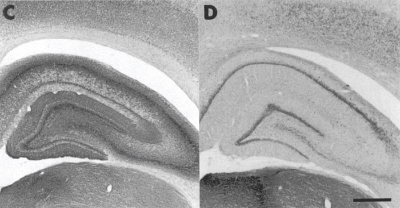
AChE-positive staining patterns in occipital and parietal cortices surrounding hippocampus. Note decrease in staining in rats treated with 192-IgG-Saporin and 5,7-DHT(D) as opposed to sham (C).
Scale bar, 750 μm
Adapted from Lehmann et al. (2002) Eur J Neurosci 15:1991-2006. Printed with permission from the Federation of European Neuroscience Societies
What about the nucleus basalis magnocellularis (NBM), another major cholinergic nucleus in the brain with cognitive implications? Again using 192 IgG-Saporin, we induced gradual lesions of NBM cholinergic neurons and ran our rats through a series of spatial tasks in which rats subjected to non-selective NBM lesions were previously shown to exhibit spatial memory impairments.7 Surprisingly, despite extensive denervation of cortical areas and utilization of a radial-maze testing protocol adding a strong temporal load to the task (trial interruption by delay of up to 6 hours), we were unable to show any working- or reference-memory deficit.7 These findings demonstrated that the basalocortical cholinergic system did not play a crucial role in such mnemonic operations. This conclusion was consolidated in another study by Lehmann, Grottick, Cassel and Higgins (in preparation), in which 192 IgG- Saporin was injected into the septal region and/or the NBM. Rats were tested in a 5-choice serial reaction test (5- CSRT) assessing visual attention and, subsequently, in a radial maze. Rats with septal lesions performed normally in the 5-CSRT, but were dramatically impaired in the radial maze. Rats with NBM lesions were markedly impaired in the 5- CSRT, but performed normally in the radial maze. Rats with both lesions were impaired in both tasks.
Our results show that 192 IgG- Saporin causing cholinergic denervation of the hippocampus (intraseptal) alters spatial memory processes, and denervation of the cortex (NBM) alters attention. At least in the hippocampus, there is evidence that attenuated serotonergic tone contributes to reduce the magnitude of spatial working memory deficits due to the cholinergic damage. Although this is probably not the only mechanism involved, we have recently found that a 5,7-DHT-induced serotonergic denervation of the hippocampus could facilitate the electrically-evoked release of acetylcholine from hippocampal slices, and that this facilitation was abolished by intrahippocampal grafts rich in serotonergic neurons.8 Thus, 192 IgG- Saporin kills cholinergic neurons and, under circumstances, cognitive functions. Depending on how the lesion is produced, it seems that killing serotonergic neurons may be an inimical collaborator or a benevolent disparager as to the cognitive malevolence of 192 IgG-Saporin.
References
- Bartus RT, Dean RL3rd, Beer B, Lippa AS (1982) Science 217: 408-414.
- Cassel JC, Jeltsch H (1995) Neurosci 69:1-41.
- Wrenn CC, Wiley RG (1998) Int J Dev Neurosci 16:595-602.
- Lehmann O, Jeltsch H, Lehnardt O, Pain L,Lazarus C, Cassel JC (2000) Eur J Neurosci 12:67-79.
- Lehmann O, Jeltsch H, Lazarus C, Tritschler L, Bertrand F, Cassel JC (2002) Pharmacol Biochem Behav 72:899-912, 2002.
- Lehmann O, Bertrand F, Jeltsch H, Morer M, Lazarus C, Will B, Cassel JC (2002) Eur J Neurosci 15:1991-2006.
- Galani R, Lehmann O, Bolmont T, Aloy E, Bertrand F, Lazarus C, Jeltsch H, Cassel JC (2002) Physiol Behav 76:75-90.
- Birthelmer A, Schweizer T, Jeltsch H, Jackisch R, Cassel JC (In Press) Eur J Neurosci.
