Saporin is a 30 kDa protein isolated from the seeds of the plant Saponaria officinalis. This pleasant resident of the banks of Southern European streams has long been known for medicinal properties, mainly due to its saponins, detergents that reside especially in the roots of the plant and which give the plant its name. It took the expertise of University of Bologna researcher Fiorenzo Stirpe to pull perhaps the most useful molecule from the plant, saporin.
Stirpe had screened several plants for ribosome-inactivating proteins. These proteins come in two different forms. One form is exemplified by the incredibly potent toxin ricin; it contains a cell-binding and internalization protein and an enzyme that upon entering the cell removes a single base from the ribosomal RNA of the large subunit of the ribosome. This enzyme action is a necessary characteristic of a ribosome- inactivating protein, cleverly termed a “RIP.” Other examples of toxins with cell-binding chains are abrin (Brooke Shields used it for her suicide in the film Blue Lagoon) and volkensin, which has been used in neuroscience research as a suicide transport agent.

Most RIPs found in plants are in the second form, without cell-binding
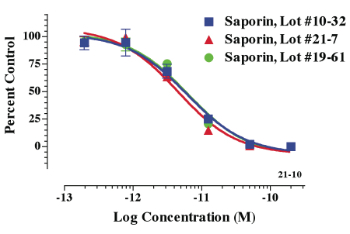
chains, and these plants are generally harmless (Fig. 1): RIPs from cucumber and asparagus are two examples. Stirpe could easily recognize the plants that had toxins, but had to screen the plants that weren’t known to be toxic. Since Saponaria had a medicinal reputation, he included it in his screening. In 1983, he published on a RIP from Saponaria, SO- 6, that was extremely active in cell-free protein synthesis inhibition, but also, in an observation that later would become very important, an unusual stability to denaturants, proteases and heat (1). This protein would become known as saporin. At that time, the RIP from ricin was widely used in targeted toxins. It had an unfortunate sensitivity to proteolytic attack when removed from its protective cell-binding chain, and this made it unable to function as a targeted toxin in cells that had high levels of proteases (2). This limited its use as a targeted toxin against, for instance, T lymphocytes, and caused considerable setbacks in the targeted toxin field. Stirpe demonstrated remarkable in vivo activities of the first targeted toxin made from saporin and, when targeted to T lymphocytes, it was devastating (3,4). Saporin was cloned by Doug Lappi and colleagues in Marco Soria’s group (5); this led to the use of recombinant saporin which Advanced Targeting Systems uses in the preclinical studies of SP-SAP. Presently, the highly active native saporin is sold by Advanced Targeting Systems in a sterile PBS solution ready for use in vitro and in vivo. Each lot is carefully assayed for full protein synthesis inhibition activity and not released unless it matches the highest levels (Fig. 2).
References
- Stirpe F, Gasper-Campani A, Barbieri L, Falasca A, Abbondanza A, Stevens WA(1983) Ribosome-inactivating proteins from the seeds of Saponaria officinalis L. (soapwort) of Agrostemma githago L. (corn cockle) and of Asparagus officinalis (asparagus) and from the latex of Hura crepitans L. (sandbox tree). Biochem J 216:617-625.
- Bilge A, Howell-Clark J, Ramakrishnan S, Press OW (1994) Degradation of ricin A chain by endosomal and lysosomal enzymes – the protective role of ricin B chain. Therapeutic Immunol 1:197-204.
- Thorpe PE, Brown ANF, Bremner JAG, Foxwell BMJ, Stirpe F (1985) An immunotoxin composed of monoclonal anti-thy 1.1 antibody and a ribosome-inactivating protein from Saponaria officinalis: potent antitumor effects in vitro and in vivo. JNCI 75:151-159.
- Siena S, Lappi DA, Bregni M, Formosa A, Villa S, Soria M, Bonadonna G, Gianni AM (1988) Synthesis and characterization of an antihuman T-lymphocyte saporin immunotoxin (OKT1-SAP) with in vivo stability into nonhuman primates. Blood 72:756-765.
- Benatti L, Saccardo MB, Dani M, Nitti GP, Sassano M, Lorenzetti R, Lappi DA, Soria M (1989) Nucleotide sequence of cDNA coding for saporin-6, a type-1 ribosome-inactivating protein from Saponaria officinalis. Eur J Biochem 183:465-470.
