Streptavidin-pHast: A readily conjugatable, pH-sensitive dye to screen for internalization
Shramm PA, Ancheta L (2019) Proceedings of the American Association for Cancer Research Annual Meeting, Atlanta GA.
Abstract
The quick and efficient screening of targeting agents that internalize effectively is vital for determining suitability as potential therapeutics. Some of the most recent successes in the treatment of cancers have been from antibodies to cell surface proteins that are in tumor cells. Examples are Sacituzumab targeting TROP-2, and Inotuzumab targeting CD22.1,2 These antibodies have more than one effect on the cancer cell, but one of the most important is that, upon binding to the cell surface antigen, the complex is internalized. Therefore, a payload conjugated to the antibody, such as a toxin, will have the desired affects to the tumor cell. Here we describe a method for the determination of internalization of cell surface molecules by targeting agents using a pH-dependent fluorescent reporter cross-linked to streptavidin. Streptavidin is a tetrameric protein (molecular weight 53 kDa in its recombinant form), with each subunit able to bind a single biotin molecule. The bond between streptavidin and biotin is rapid and essentially non-reversible, unaffected by most extremes of pH, organic solvents, and denaturing reagents. It is the strongest known noncovalent biological interaction (Ka = 1015 M-1) between protein and ligand. A variety of molecules, including lectins, proteins, and antibodies, can be biotinylated and reacted with streptavidin-labeled probes or other detection reagents for use in biological assays. The fluorescence from this reporter increases intensity as the pH of its surroundings becomes more acidic, as evident when exposed to the environment inside a cell (thereby providing evidence of internalization). Here we describe methods that can be used to explore candidates as therapeutics in a quick and reproducible manner.
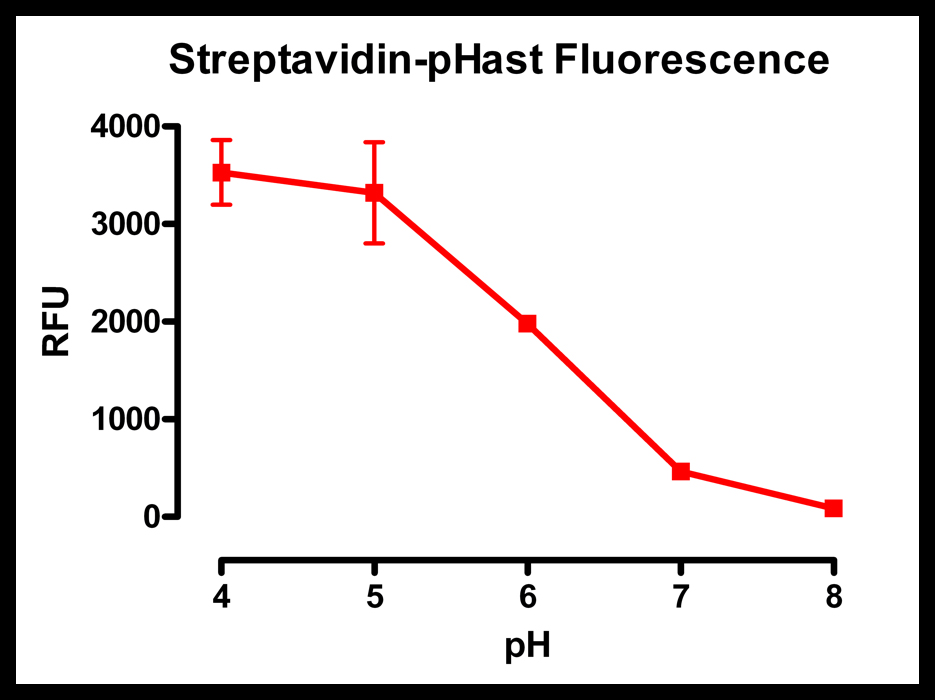
Streptavidin-pHast was diluted 1:100 in 50 mM potassium phosphate with varying pH levels. The more acidic pH shows a large amount of fluorescence, while the basic pH shows almost no fluorescence.
Plates read on a Spectra Max Gemini EM (Ex: 532nm/Em: 560nm).
Introduction
The Streptavidin-pHast compound consists of two components, (1) recombinant streptavidin in its tetrameric form and (2) a fluorescent dye (pHast) that carries a relative excitation and emission spectra of 532 nm and 560 nm
A similar conjugate, Fab-pHast (Cat. PH-01; Advanced Targeting Systems), a chemical conjugate of a monovalent antibody and the pHast dye, has already been created and used to determine internalization of antibodies and lysosomal trafficking3. However, this conjugate is only applicable for species specific antibodies.
Streptavidin has been used as a common conjugation tool to create immunotoxins with antibodies4 as well as a tool to screen for internalization via cytoxox assays using Streptavidin-ZAP5. Antibodies, lectins, peptides, and other targeting proteins can be biotinylated and therefore screened for targeting abilities. In order to make a more robust pHast-associated screening tool, Streptavidin-pHast has been synthesized and used here with various proteins and applications.
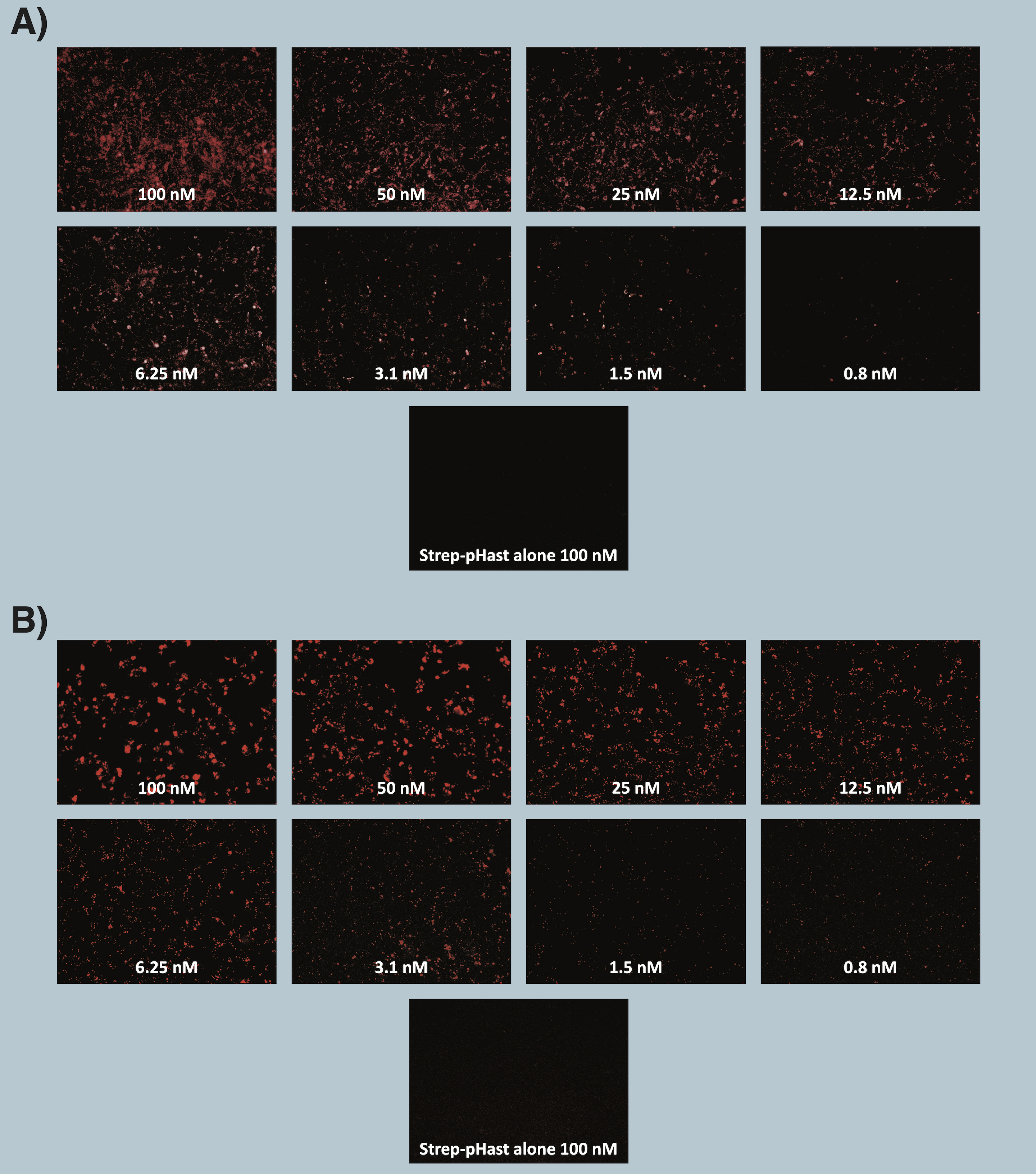
Cells were plated at 20,000 cells/well in a 96-well plate and allowed to adhere overnight. Varying concentrations of Antibody-pHast conjugate were incubated on cells overnight to allow maximum internalization, although internalization can be detected in a few hours. Cells were analyzed on a Leica microscope under 10X magnification using a Y3 filter cube. Media was replaced with PBS prior to imaging.
A) Immunocytochemistry images of biotinylated 192-IgG, an antibody to the rat p75 receptor, conjugated and illuminated with Streptavidin-pHast on 7H6 cells.
B) Immunocytochemistry images of biotinylated mu-p75, an antibody to the mouse p75 receptor, conjugated and illuminated with Streptavidin-pHast on NG6 cells.
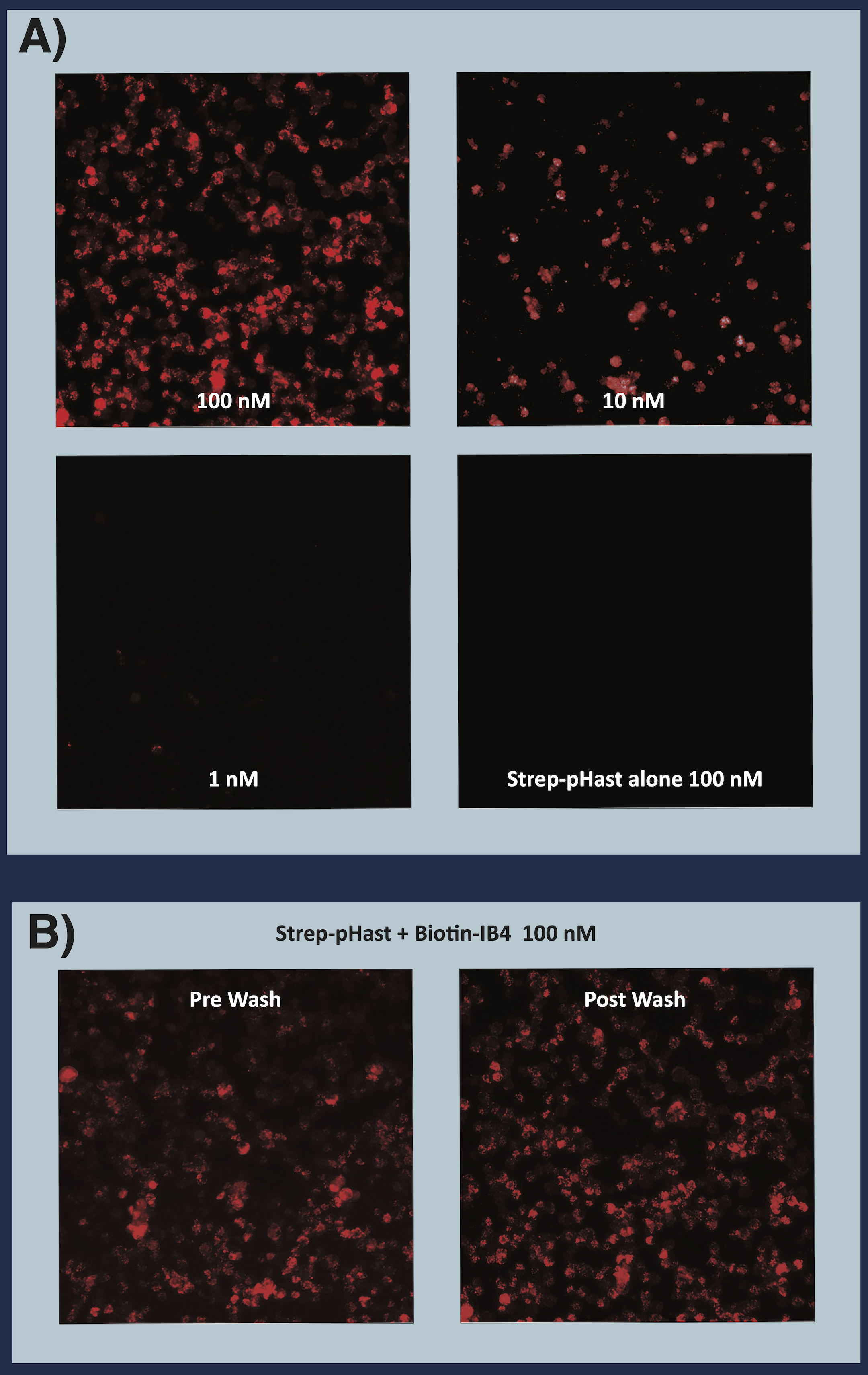
A) Biotinylated rIB4 (recombinant Isolectin B4) illuminated with Streptavidin-pHast. KNRK cells were plated at 20,000 cells/well in a 96-well plate and allowed to adhere overnight. Biotinylated IB4 was incubated at RT with Streptavidin-pHast for 20 minutes. The conjugate and cells were incubated overnight to allow maximum internalization, although internalization can be detected in a few hours. Cells were analyzed on a Leica microscope under 10X magnification using a Y3 filter cube.
B) This image shows the relative lack of difference in fluorescence when replacing the media with Streptavidin-pHast. Highlighting that Streptavidin-pHast will only fluoresce when internalized by the cell, and that while washing or changing the media will remove a small amount of background, it is not always necessary.
Discussion
Streptavidin-pHast is a novel tool designed for quick and efficient screening of biotinylated targeting agents. These compiled data support the use of Streptavidin-pHast in various applications and the different methods available to visualize the construct.
One application described in detail is the use of Streptavidin-pHast in immunocytochemistry (ICC). ICC data demonstrate that even at low concentrations (0.8 nM of Streptavidin-pHast mixed equimolar with a biotinylated targeting agent), confirmation of internalization can still be seen. Reaction time of the targeted pHast conjugate was found to be dependent not only on the amount of conjugate used, but also on the number of cells. At higher magnifications, punctate structures have been observed inside the cells, which indicates that the pHast conjugate has been internalized and is in the acidic endosomes and lysosomes of the cell.
In all three applications tested — ICC, flow cytometry, and fluorescent plate reader — the cells and targeted pHast conjugate were allowed to react overnight to allow for maximum internalization, however fluorescence-confirming internalization can be detected in a just a few hours in all assays. The data here demonstrate the potential variables in sensitivity in regards to equipment when visualizing data. Similar results were seen across the board, but subtle differences were observed, especially in the lower concentration levels. This was particularly true in the ability to see a difference at 0.8 nM between controls and test samples in ICC.
Another potential application is a time-based kinetic assay depicting an increase in fluorescence over time. This type of assay has been shown to be an effective way to screen for lysosomal trafficking3 using a pHast conjugate.
Overall, these data demonstrate that a biotinylated targeting agent, either antibody or peptide, could be internalized by the cell via receptor-mediated endocytosis and depicts the potential usefulness of the ability to screen a wide range of proteins for the ability to target, enter, and deliver a payload to a cell.
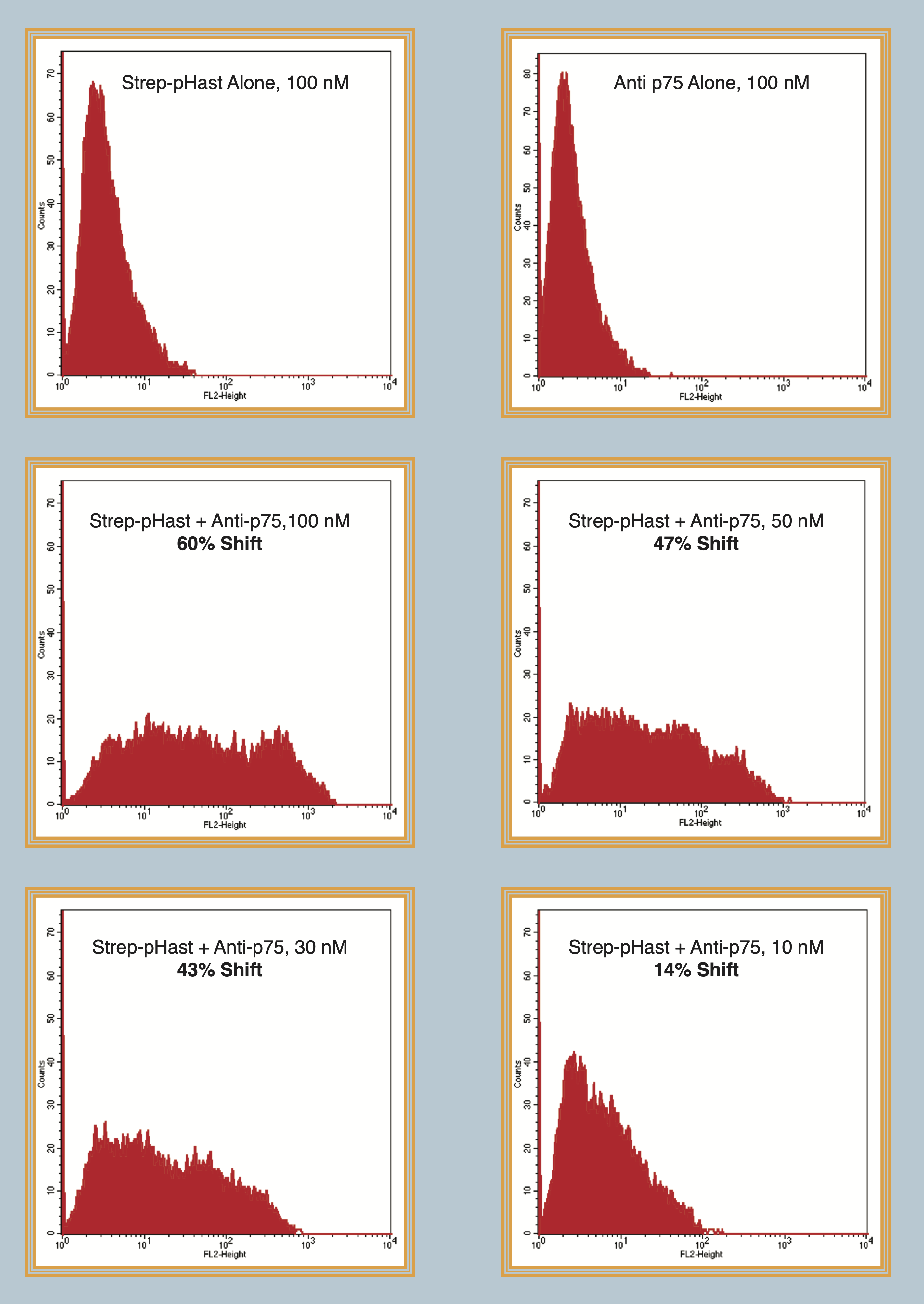
Flow cytometry data of biotinylated mu-p75, an antibody to the mouse p75 receptor, conjugated and illuminated with Streptavidin-pHast. NG6 cells were plated at 100,000 cells/well in a 6-well plate and allowed to adhere overnight. Varying concentrations of Antibody-pHast conjugate were incubated on cells overnight to allow maximum internalization, although internalization can be detected in a few hours. Cells were analyzed on a BD FACscan. Cells were washed with flow buffer, PBS/2% FBS, prior to analysis. This demonstrates that Streptavidin-pHast can be used in flow cytometry-based assays.
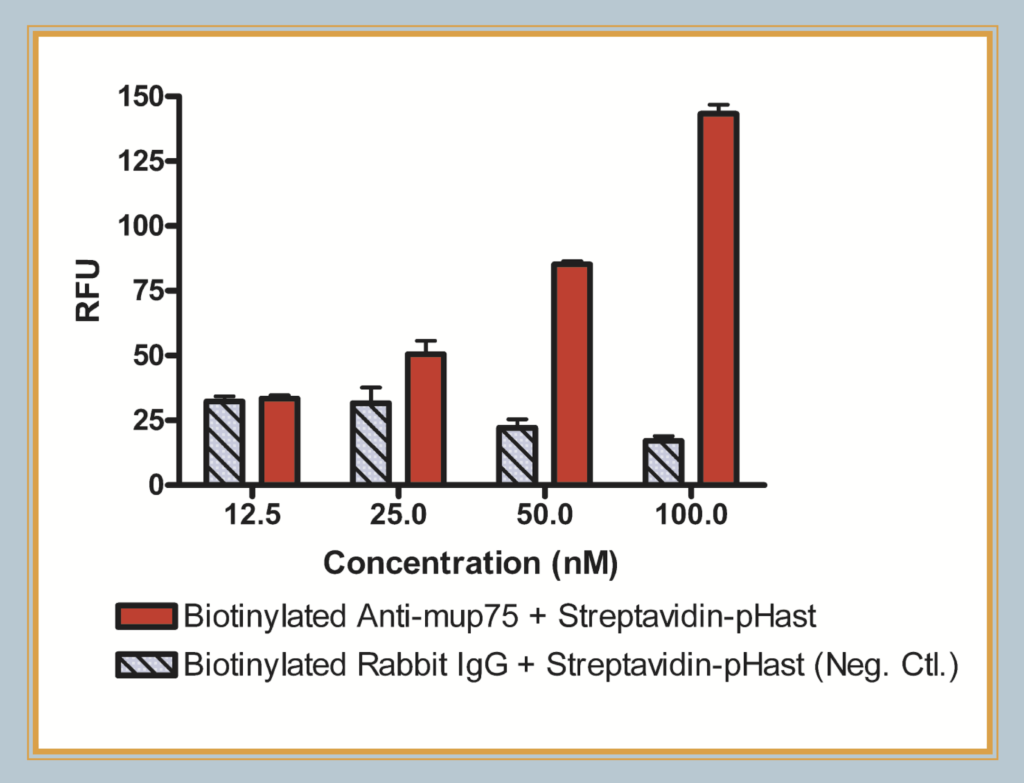
Fluorescent plate reader data of biotinylated mu p75, an antibody to the mouse p75 receptor, conjugated and illuminated with Streptavidin-pHast. Cells were plated at 20,000 cells/well in a 96-well plate and allowed to adhere overnight. Varying concentrations of Antibody-pHast conjugate were incubated on cells overnight to allow maximum internalization, although internalization can be detected in a few hours. This graph demonstrates the potential of using Streptavidin-pHast in a plate reader-based assay to perform high- throughput screening of antibody efficacies.
Plates read on a Spectra Max Gemini EM (Ex: 532nm/Em: 560nm).
Conclusion
- Streptavidin-pHast, in presence of acidic pH, has shown high levels of fluorescence.
- Streptavidin-pHast is highly fluorescent when internalized by cells presumably due to the acidic endosomal and lysosomal lumen pH.
- This new conjugable tool widens the potential platforms available to screen for internalization
- The speed of which internalization can be visualized is considerably faster (1 hr) versus traditional internalization assays (3 days).
- Determining specificity, binding and internalization has been made easier through immunocytochemistry and biotinylated targeting.
- Results describe a plate-based assay with consistent, reproducible data screening biotinylated targeting agents for their receptor-mediated internalization properties.
- More work needs to done in regards to the effects of abnormal pH in the extracellular and intracellular environments on fluorescence.
References
- Zangardi ML, Spring LM, Nagayama A, Bardia A (2019) Sacituzumab for the treatment of triple-negative breast cancer: the poster child of future therapy? Expert Opin Investig Drugs 28(2):107-112. doi: 10.1080/13543784.2019.1555239 PMID: 30507322
- Lamb YN (2017) Inotuzumab ozogamicin: First global approval. Drugs 77(14):1603-1610. doi: 10.1007/s40265-017-0802-5 PMID: 28819740
- Kinneer K, Meekin J, Tiberghien AC, Tai YT, Phipps S, Kiefer CM, Rebelatto MC, Dimasi N, Moriarty A, Papadopoulos KP, Sridhar S, Gregson SJ, Wick MJ, Masterson L, Anderson KC, Herbst R, Howard PW, Tice DA (2018) SLC46A3 as a potential predictive biomarker for antibody-drug conjugates bearing noncleavable linked maytansinoid and pyrrolobenzodiazepine warheads. Clin Cancer Res 24(24):6570-6582. doi: 10.1158/1078-0432.CCR-18-1300 PMID: 30131388
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang L-P, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G (2011) Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475:226-230. doi: 10.1038/nature10169 PMID: 21753853
- Kohls M (2006) Evaluate Potential Targeting Molecules. Nature Methods PMID: 909090
