A rapid, pH-sensitive screening method to detect internalization of cell surface markers for development of antibody-based pharmaceuticals to treat brain tumors.
Shramm PA, Ancheta L, Higgins D, Lappi DA (2017) Society for Neuroscience, Washington, DC.
Abstract
Some of the most recent successes in the treatment of cancers have been from antibodies to cell surface proteins that cause tumor cell proliferation. Examples are cetuximab (antigen: EGFR) approved for colorectal cancer, and Trastuzumab (ERBB2) for breast cancer. These antibodies have more than one effect on the cancer cell, but one of the most important is that, upon binding to the cell surface antigen, the complex is internalized by antibody-mediated internalization. As such, the down-regulated cell surface protein no longer plays a role in cancer cell division. Despite the blood brain barrier challenging systemic treatment for brain tumors, intracerebroventricular injection can produce similar results. For example, Gholamin et al.1 and Kang et al.2 reported down-regulation of brain tumor mitogenic agents through antibody-mediated endocytosis. The quick and efficient screening of antibodies that internalize effectively is vital for determining suitability of an antibody as a therapeutic targeting agent. Here we describe a method for the efficient determination of internalization of cell surface molecules by antibodies using a pH-dependent fluorescent reporter cross-linked to a secondary antibody in a plate-based assay with visualization of internalization in hours. This conjugate is comprised of an affinity-purified monovalent secondary antibody against both the heavy and light chain of human or mouse IgG conjugated to a pH-dependent fluorescent reporter. The fluorescence from this reporter increases intensity as the pH of its surroundings becomes more acidic, as evident when exposed to the environment inside a cell (thereby providing evidence of internalization). A successful assay protocol has been developed to provide an EC50 by way of a fluorescence-detecting plate reader, which can be used to explore antibody candidates as therapeutics in a quick and reproducible manner.
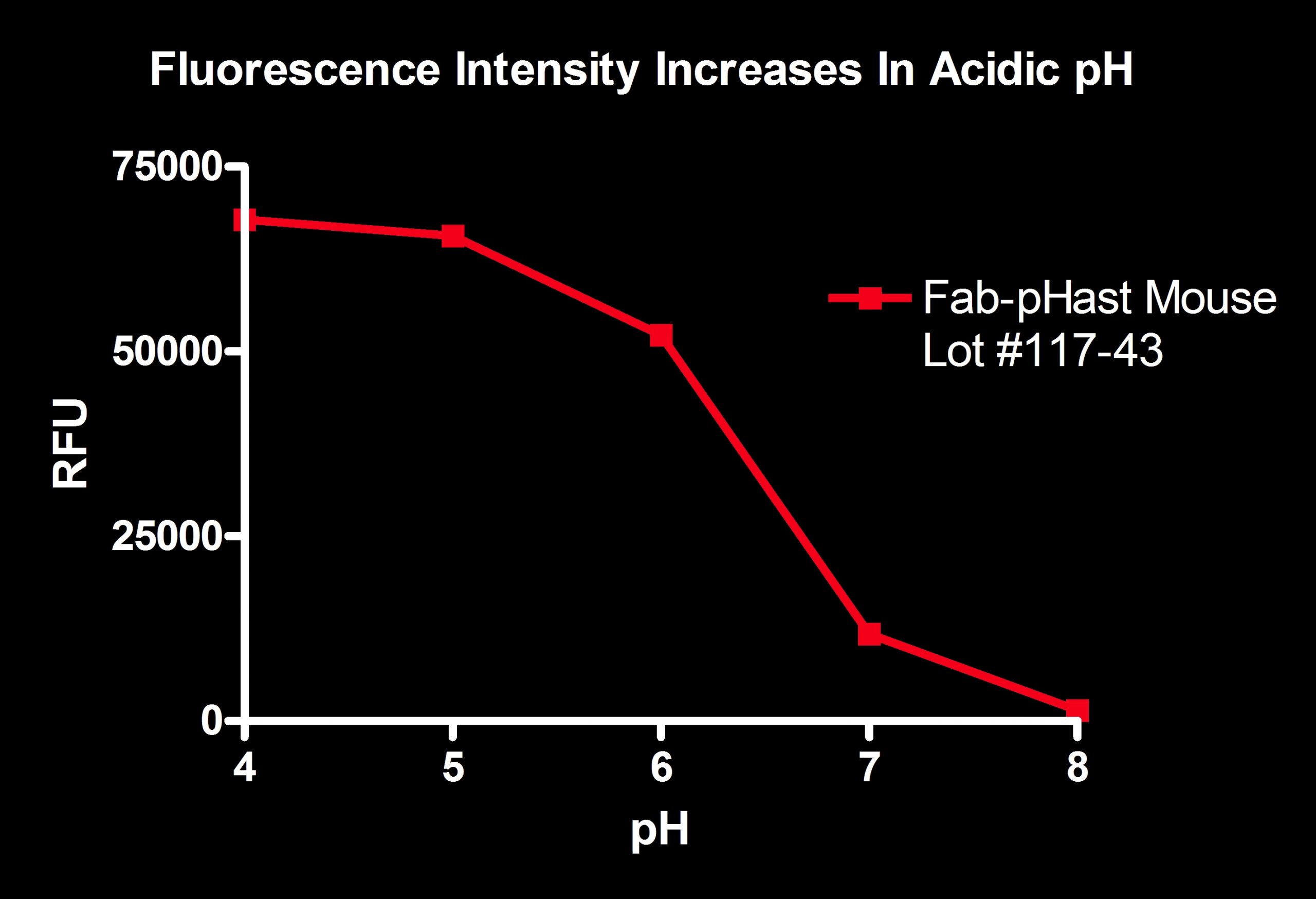
Fab-pHast Mouse was diluted 1:100 in 50 mM potassium phosphate with varying pH levels. The more acidic pH shows a large amount of fluorescence, while the basic pH shows almost no fluorescence.
Plates read on a Spectra Max Gemini EM (Ex: 532nm/Em: 560nm).
Methods
Fab-pHast is a chemical conjugate of a monovalent antibody and a pH-dependent fluorescent reporter. The antibody fragments used to make Fab-pHast are affinity-purified polyclonal antibodies. The Fab fragment is composed of one constant and one variable domain of each of the heavy and light chain of an IgG. The antibody fragment used in this product will cross-react across immunoglobulin classes and subclasses of the same species as they share the same light chain (either kappa or lambda). The pHast fluorescent dye has an excitation wavelength of 532 nm with an emission maxima at 560 nm.
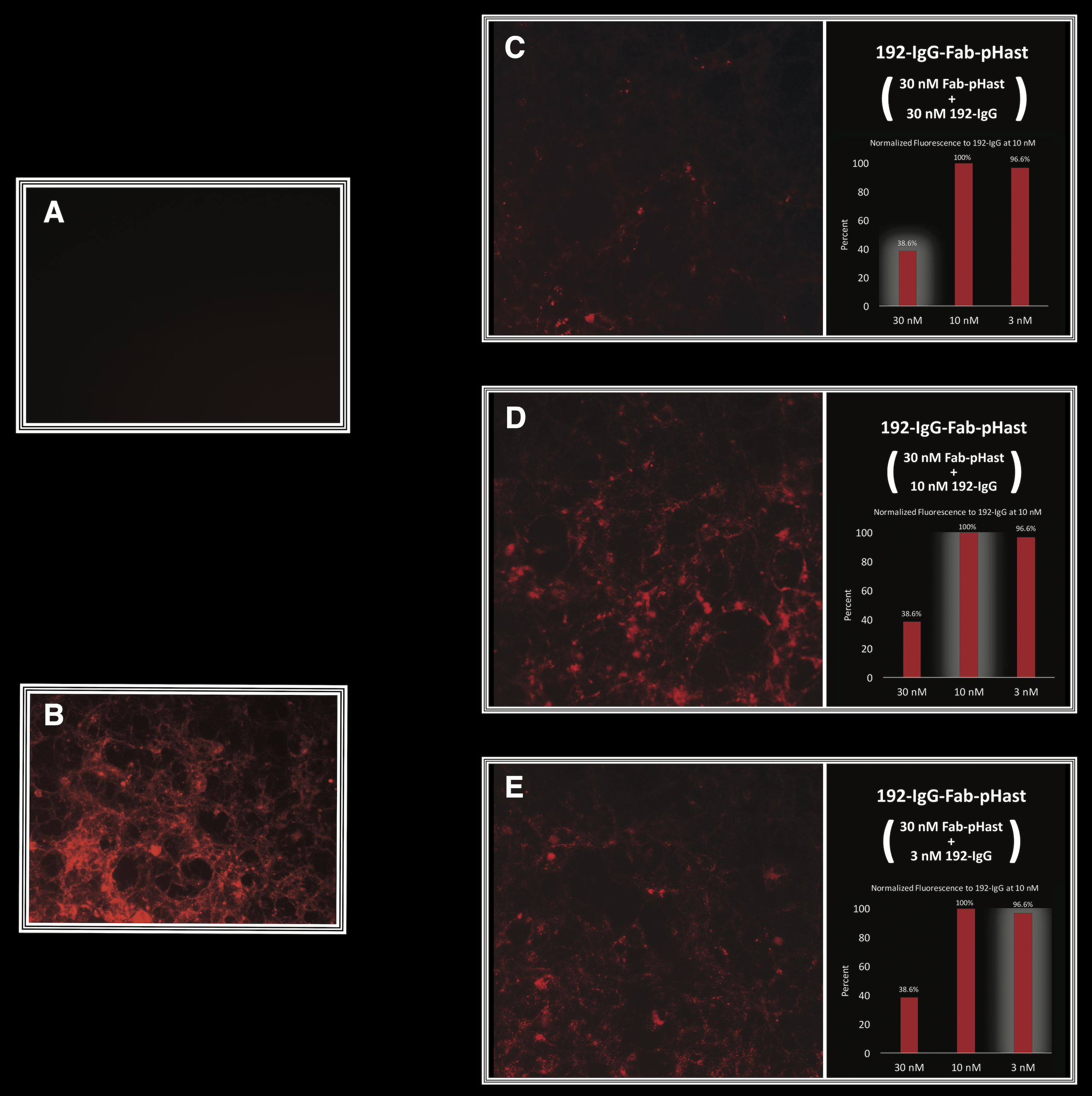
HEK-293 cells, transfected with the p75 receptor, were plated at 20,000 cells/well in a 96-well plate and allowed to adhere overnight. Different amounts of antibody were incubated at RT with 30 nM of Fab-pHast. Cells were incubated overnight to allow maximum internalization, although internalization can be detected in a few hours. Cells were analyzed on a Leica microscope under 20X magnification using a Y3 filter cube. The bar graphs show the relative fluorescence of varying concentrations of antibody.
A) Cells incubated with Fab-pHast alone, showing no fluorescence.
B) Cells incubated with a positive control, 192-IgG-Cy3 (a conjugate of the antibody and Cy3).
C) Cells incubated with 30 nM of Fab-pHast and 30 nM of 192-IgG, showing the least amount of fluorescence. D) Cells incubated with 30 nM of Fab-pHast and 10 nM 192-IgG, showing the greatest amount of fluorescence. E) Cells incubated with 30 nM of Fab-pHast and 3 nM of 192-IgG.
Note: For a discussion of the role of NGF in brain and nervous system cancers, see the SfN 2017 poster by Ancheta et al. 612.11/SS46).
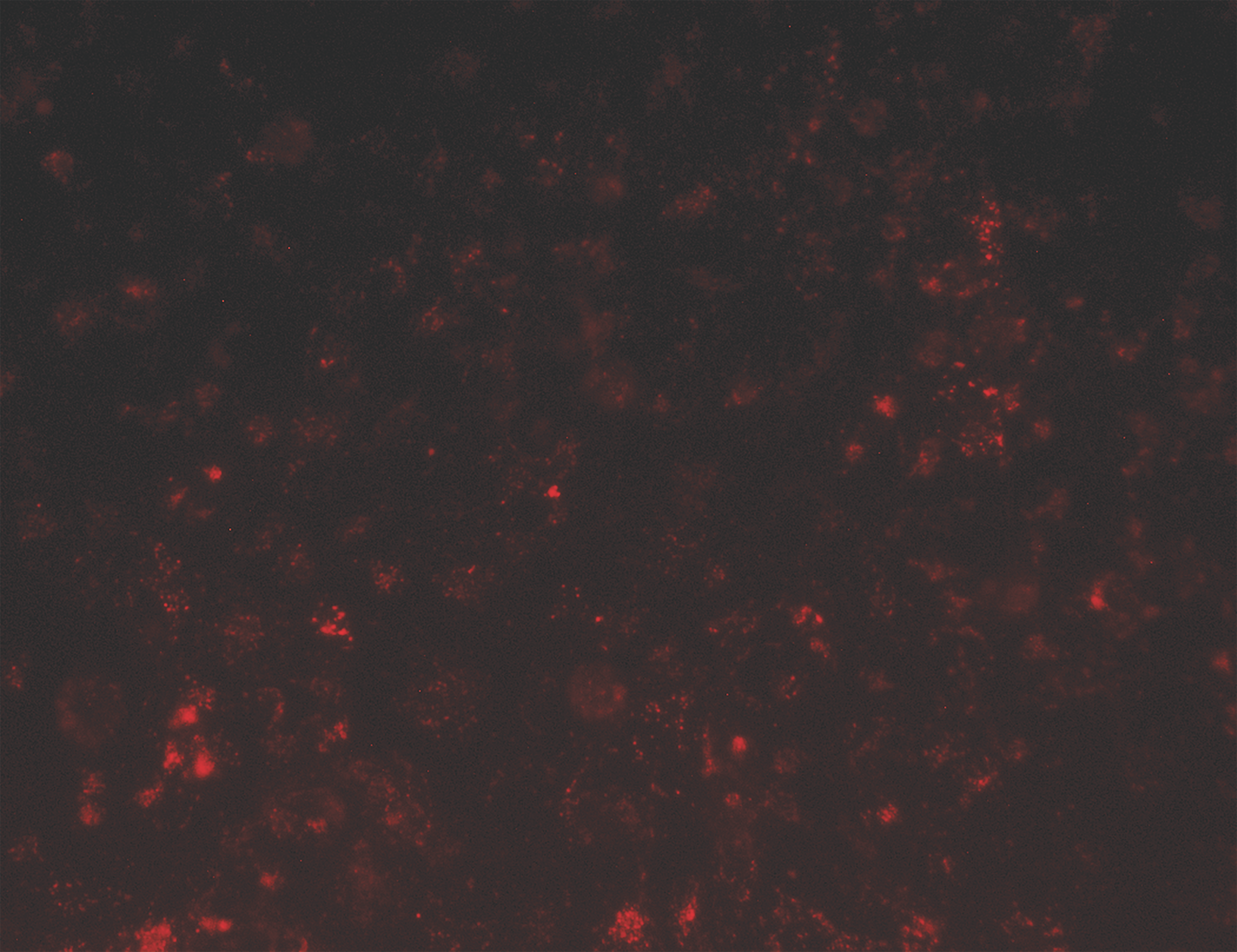
The receptor for Basigin-2 is a known tumor marker and an antibody directed against this receptor could be a potential cancer treatment.3 NCI-ADR/RES cells were plated at 20,000 cells/well in a 96-well plate and allowed to adhere overnight. 2.5 nM of antibody was incubated at RT with 50 nM of Fab-pHast. Cells were incubated overnight to allow maximum internalization, although internalization can be detected in a few hours. Cells were analyzed on a Leica microscope under 20X magnification using a Y3 filter cube.
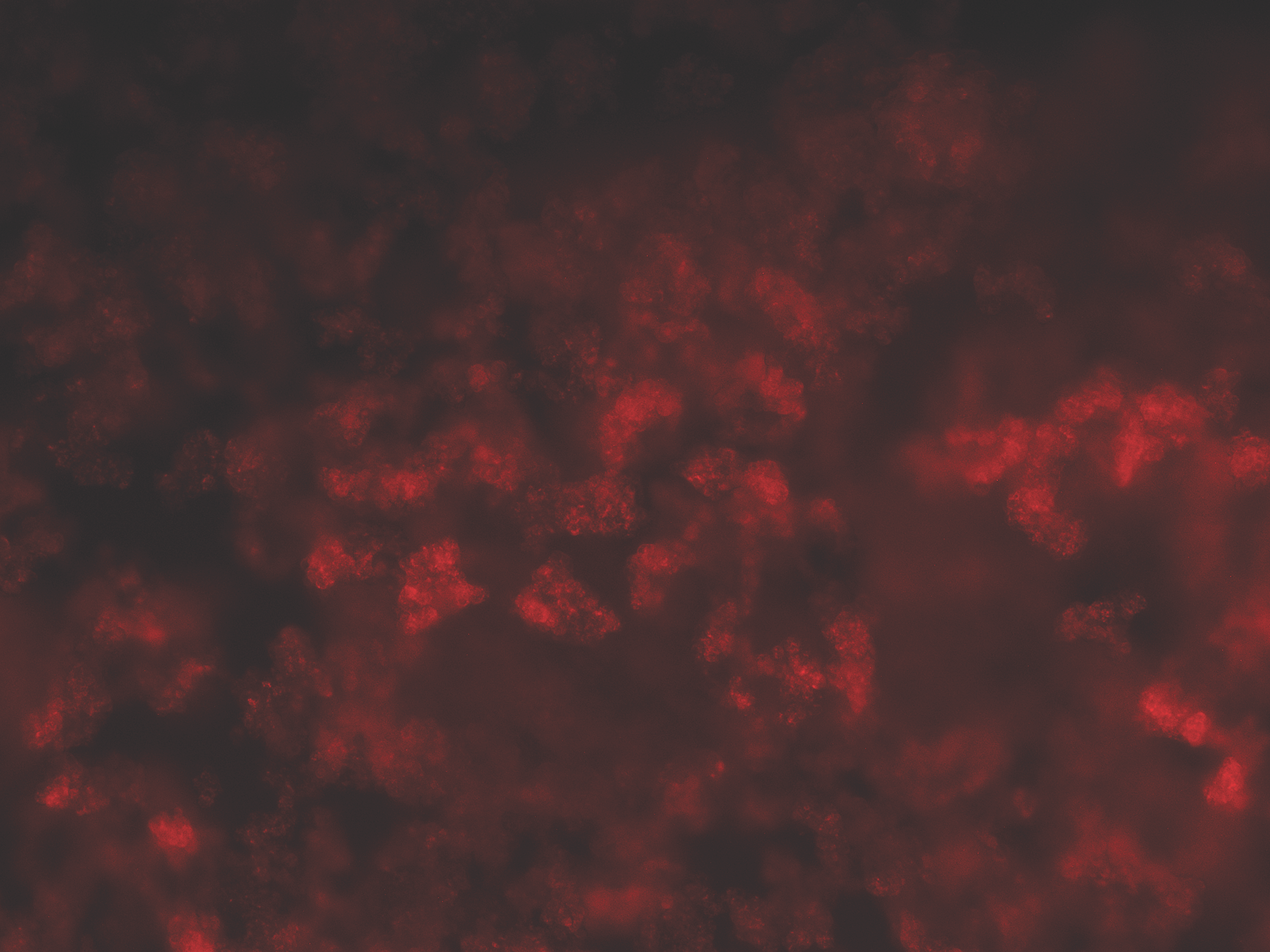
PC12 cells were plated at 20,000 cells/well in a 96-well plate and allowed to adhere overnight. 10 nM of antibody was incubated at RT with 50 nM of Fab-pHast. Cells were incubated overnight to allow maximum internalization, although internalization can be detected in a few hours. Cells were analyzed on a Leica microscope under 20X magnification using a Y3 filter cube.
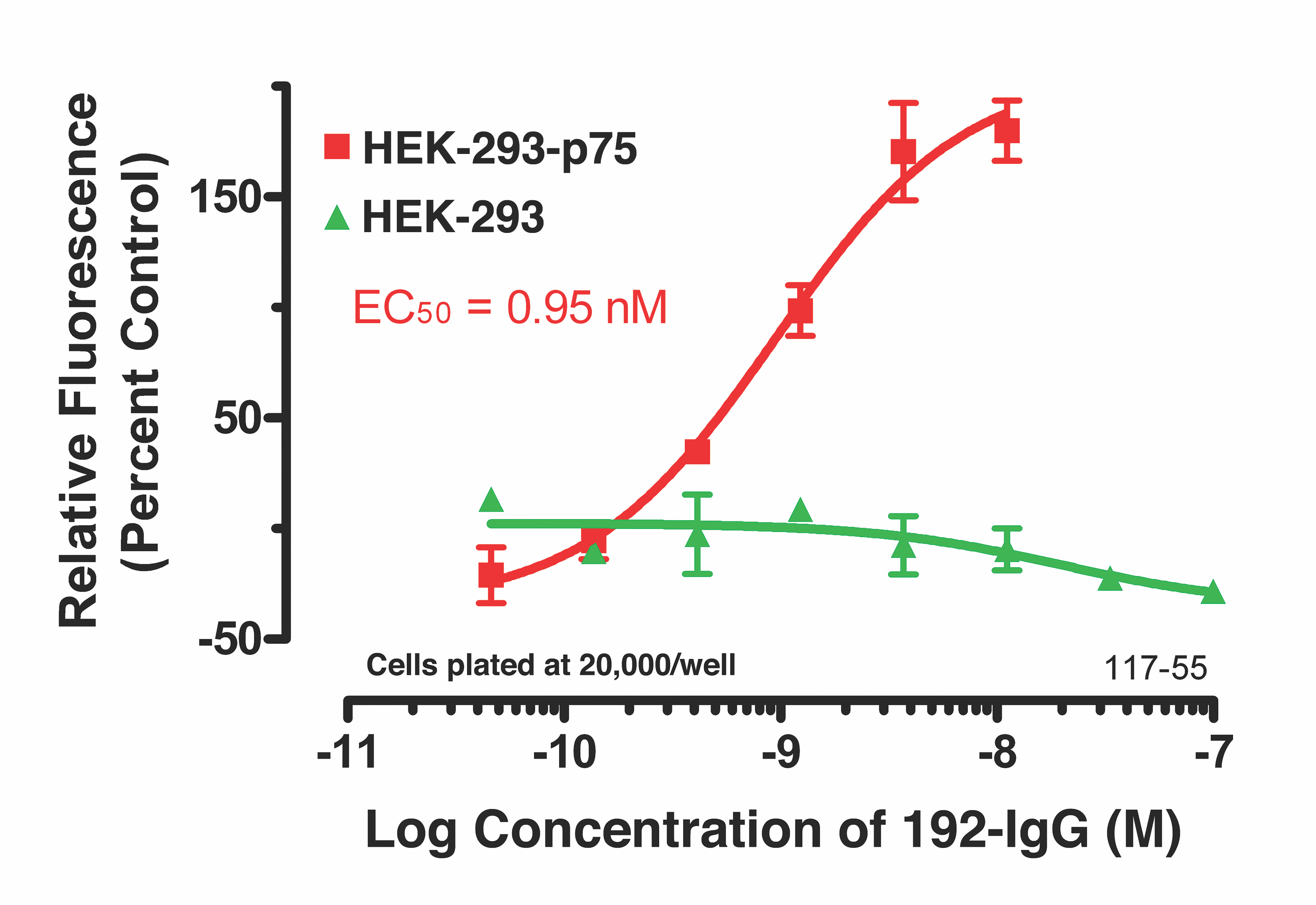
Parental HEK-293 cells, and HEK-293 cells transfected with the p75 receptor, were plated in a 96-well plate overnight. Titrated 192-IgG antibody was incubated at RT with 50 nM of Fab-pHast Mouse for 20 minutes prior to addition to cells. Plates were incubated overnight to allow maxi- mum internalization. Plates were read on a Spectra Max Gemini EM (Ex: 532nm/Em: 560nm). Data analysis was done by PRISM (Graph- Pad, San Diego).
Conclusions
- Fab-pHast is highly fluorescent in acidic pH.
- Fab-pHast is highly fluorescent when internalized by cells because of the endosomal and lysosomal lumen pH.
- We have made a new secondary conjugate to evaluate the potential of a primary antibody to internalize.
- This secondary conjugate is a much faster alternative to traditional antibody internalization assays. Internalization can be detected in as little as an hour. Maximal fluorescence occurs after an overnight incubation.
- We have made a simple way to visualize specificity, binding, and internalization of an antibody via immunocytochemistry.
- We have made an easy and reproducible plate based assay that enables antibodies to be screened based on their receptor-mediated internalization properties.
Note: Abnormal pH in extracellular and intracellular environments may affect the fluorescence of Fab-pHast.
References
- Gholamin S et al. (2017) Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 9(381):eaaf2968. doi: 10.1126/scitranslmed.aaf2968 PMID: 28298418
- Kang BR, Yang SH, Chung BR, Kim W, Kim Y (2016) Cell surface GRP78 as a biomarker and target for suppressing glioma cells. Sci Rep 6:34922. doi: 10.1038/srep34922. PMID: 27713511
- Ancheta LR, Lappi DA (2011) Basigin-2 (EMMPRIN), a prognostic marker, is a dynamic portal of entry into cancer cells. Cancer Res 71(8):5218. Proceedings of the American Association for Cancer Research Annual Meeting, Orlando, FL. doi: 10.1158/1538-7445.AM2011-5218
- Ancheta L, Shramm PA, Lappi DA (2017) Method for screening neuronal tumor cell surface markers for high specificity and rapid internalization as potential oncologic treatments. Neuroscience 2017 Abstracts 612.11 / SS46. Society for Neuroscience, Washington, DC.
