Antibody Drug Conjugates to Treat Cancer
Antibody Screening for Cancer Research and Drug Development
There is a growing need for therapeutics for cancer, autoimmune diseases, blood diseases and Alzheimer’s disease. Due to their ability to bind to a specific target antigen, monoclonal antibodies are increasingly being screened for therapeutic drug development. In May 2021, the FDA approved the 100th monoclonal antibody since 1986. Antibody therapeutics account for more than one fifth of the FDAs new drug approvals each year [1].
“The global therapeutic monoclonal antibody market was valued at approximately $115.2 billion in 2018 and is expected to generate revenues of $300 billion by 2025. As of December 2019, 79 therapeutic monoclonal antibodies have been approved by the US FDA”[2].
The Importance of Screening Antibodies
There are a couple of very important reasons to screen antibodies.
- To discover useful antibodies for therapeutic and research purposes
- To determine specificity, functional binding, internalization, and EC50 determination
Advanced Targeting Systems (ATS) has screening tools to recognize any antibody with result times ranging from 24-72 hours.
Product Screening Capabilities
| Multiple Antibodies | Antibody Subtypes | Antibody Species | Time to Results | |
|---|---|---|---|---|
| ZAP Internalization Kits | X | IgG, IgM, IgY All biotinylated antibodies | Human, Mouse, Rat, Rabbit, Alpaca, Chicken, Goat, Guinea Pig, Custom Requests | 72 hours |
| pHast Conjugates (pH-based) | X | All biotinylated antibodies | Human, Mouse, Rat, Rabbit, Alpaca, Chicken, Goat, Guinea Pig, Custom Requests | 24 hours |
The ZAP technology provides the tools to attach your antibody to Saporin. Saporin is a ribosome-inactivating protein that has no way to enter a cell on its own (except through bulk-phase endocytosis). When attached to your primary antibody, it will be taken inside any cell that recognizes your antibody on the cell surface. Once inside, Saporin inactivates the ribosomes and provides a definitive result of how specific your antibody is to cells a particular cell surface marker.

The pHast technology works in a similar manner to attach your antibody to a pH-dependent fluorescent reporter. This fluorescent reporter will increase intensity as the pH of its surroundings becomes more acidic, as evident when exposed to the environment inside a cell. A successful assay will provide an EC50 by way of a fluorescence detecting plate reader, illuminating your lead antibody candidates.
Immunotoxins for Cancer Research
Immunotoxins made with Saporin are robust tools, especially in the fields of cancer [3,4]. As studies in these fields expand, finding a modular way of creating screening tools is more desirable. Particularly in drug development, large numbers of antibodies need to be screened for suitability; choosing the right antibody (or other targeting agent) is key. Screening can be prohibitively expensive in both cost and time. That is why Advanced Targeting Systems developed the ZAP technology. ZAP conjugates are non-targeted Saporin conjugates that “piggyback” on to your primary targeting agent (biotinylated material or antibody).
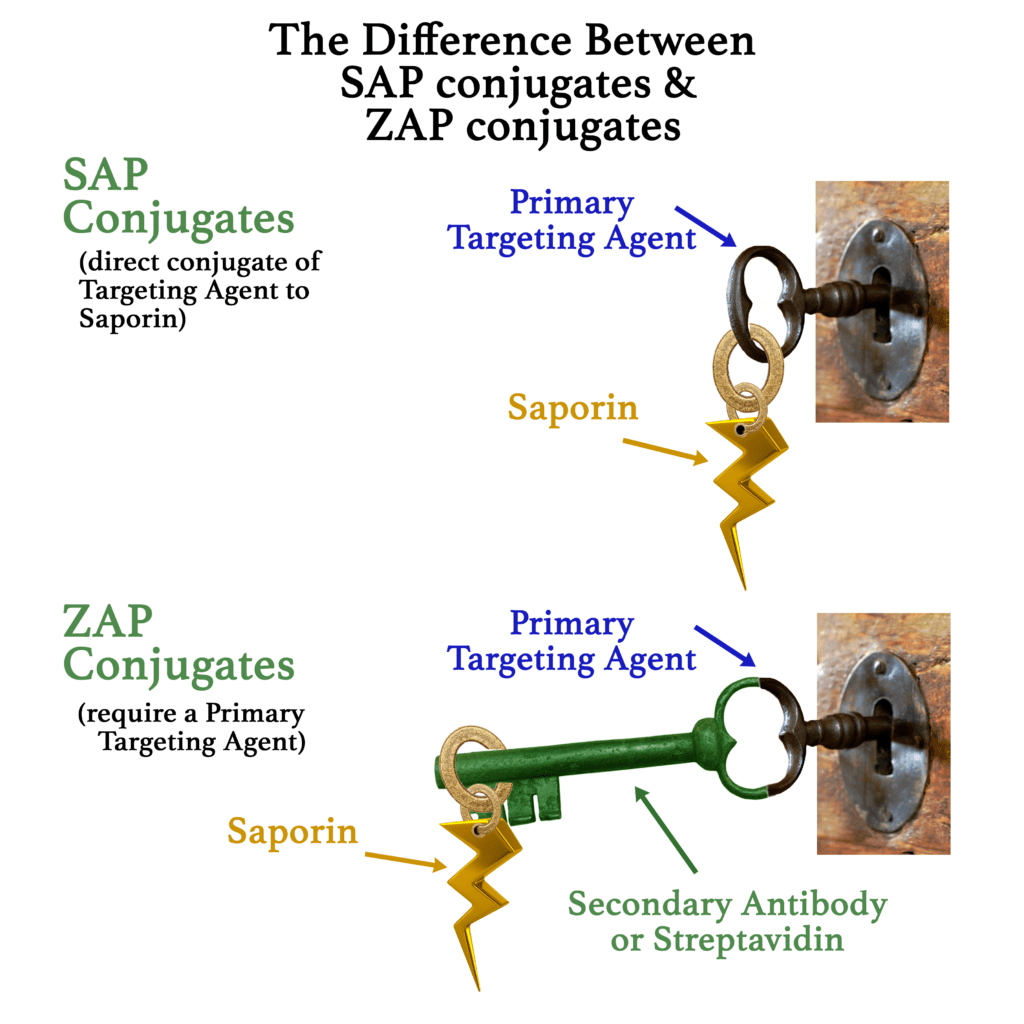
Targeted conjugates are widely used to escort payloads to specific cell populations in vitro and in vivo for both basic research and drug development. A molecule that targets the extracellular marker of choice (a Targeting Agent) must be identified, produced, and specificity must be characterized. Desirable traits of a Targeting Agent include high specificity and rapid internalization. The Targeting Agent can be an antibody, peptide, protein, aptamer, or any other molecule that recognizes a cell-surface marker. Antibodies often make the best targeting agent, and the choice of the correct antibody is crucial to the specificity and performance of payload delivery.
References
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov 2021, 20, 491-495, doi:10.1038/d41573-021-00079-7.
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020, 27, 1, doi:10.1186/s12929-019-0592-z.
- Giansanti, F.; Flavell, D.J.; Angelucci, F.; Fabbrini, M.S.; Ippoliti, R. Strategies to Improve the Clinical Utility of Saporin-Based Targeted Toxins. Toxins (Basel) 2018, 10. doi:10.3390/toxins10020082
- Polito, L.; Bortolotti, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Saporin-S6: a useful tool in cancer therapy. Toxins (Basel) 2013, 5, 1698-1722. doi:10.3390/toxins5101698
