Contributed by Robert M. Caudle, Ph.D.
University of Florida College of Dentistry, Gainesville, FL 32610
A substantial amount of work with Advanced Targeting Systems’ substance P/saporin conjugate (SP-SAP, Cat. #IT-07) has demonstrated that neurokinin-1 (NK1) receptor-expressing neurons in the spinal cord and brain stem are necessary for the full expression of central sensitization following a peripheral injury [1-7]. Eliminating these neurons with SP-SAP suppresses the hyperalgesia (an enhanced sensation of pain) and allodynia (pain to normally non-painful stimuli) associated with the injury, yet, amazingly, leaves normal pain sensation intact. These findings indicate that the NK1 receptor marks an important set of pain processing neurons in the central nervous system and that alterations in their function lead to an enhancement of the pain sensations experienced by an individual.
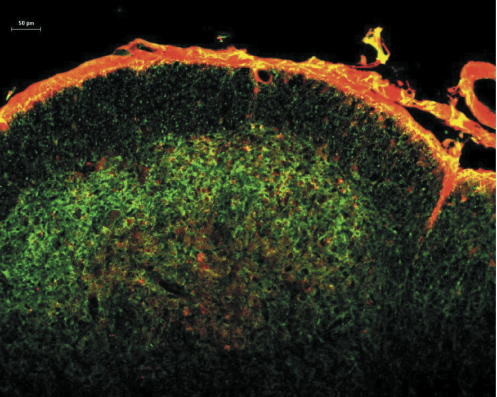
Studies on the molecular properties of central neurons during chronic pain conditions suggest that activation of several kinases through either enhanced calcium entry into the cells, enhanced internal calcium release or through the stimulation of cAMP production leads to the sensitization of these neurons [8-22]. These studies provide valuable information on the function of the NK1 receptor-expressing neurons, but they require an injury to produce the sensitization, which involves the activation of a large number of molecular pathways simultaneously. To selectively stimulate the cAMP pathway we chose to use cholera toxin. Cholera toxin stimulates cAMP production by ADP-ribosylating Gs, which then stimulates adenylate cyclase [23]. By chemically coupling substance P to the C-terminus of the A subunit (catalytic subunit) of cholera toxin (SP-CTA, Cat. #IT-39) we were able to target the CTA specifically to NK1 receptor-expressing cells [24]. Figure 1 illustrates the uptake of SP-CTA into rat cervical dorsal horn neurons following an intracisternal injection. The section is co-labeled with antibodies to NK1 receptors demonstrating co-localization of the toxin and receptors. The conjugate was not observed in any neurons or cells that did not express NK1 receptors.
In a series of experiments we tested SP-CTA on behavior following intrathecal administration to rats. Doses from 0.3 to 3 µg produced significant sensitization to thermal stimuli on the hind paws of rats 24 hours following the injections. Interestingly, with higher doses the sensitization appeared to be suppressed [24]. These findings suggested that descending inhibitory pathways were activated by the SP-CTA-induced sensitization. Preliminary data indicate that the opioid antagonist naloxone can suppress the descending inhibitory control induced by SP-CTA, exposing profound hypersensitivity to thermal stimuli (data not shown). These findings support the hypothesis that descending control systems are activated by the actions of SP-CTA on spinal cord and brain stem NK1 receptor-expressing neurons.
In summary, SP-CTA is an exciting new tool to examine the function of neurons that possess neurokinin receptors in the central nervous system. The agent selectively enters these neurons through the NK1 receptors and stimulates the production of cAMP for up to three days [24]. As revealed by our studies novel cell functions can be found through this form of selective neuronal stimulation.
References: (back to top)
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA (2002) Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci 22(20):9086-9098.
- Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA (1997) Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278:275-279.
- Simons CT, Gogineni AG, Iodi Carstens M, Carstens E (2002) Reduced aversion to oral capsaicin following neurotoxic destruction of superficial medullary neurons expressing NK-1 receptors. Brain Res 945:139-143.
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH (2002) Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci 5(12):1319-1326.
- Vierck CJ, Kline RH, Wiley RG (2003) Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience 119(1):223-232.
- Wiley RG, Lappi DA (1997) Destruction of neurokinin-1 receptor expressing cells in vitro and in vivo using substance P-saporin. Neurosci Lett 230:97-100.
- Wiley RG, Lappi DA (1999) Targeting neurokinin-1 receptor-expressing neurons with [Sar9, Met(O2)11] substance P-saporin. Neurosci Lett 277(1):1-4.
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ (2004) Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci 20(2):375-384.
- Caudle RM, Perez FM, Del Valle-Pinero AY, Iadarola MJ (2005) Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol Pain 1:25.
- Chen L, Huang LY (1992) Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature 356(6369):521-523.
- Gao X, Kim HK, Chung JM, Chung K (2005) Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain 116(1-2):62-72.
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K (2002) Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci 22(14):6208-6217.
- Ji RR, Woolf CJ (2001) Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis 8(1):1-10.
- Lin Q, Peng YB, Willis WD (1996) Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. J Neurosci 16(9):3026-3034.
- Ren K, Dubner R (1999) Central nervous system plasticity and persistent pain. J Orofac Pain 13(3):155-163.
- Riedel W, Neeck G (2001) Nociception, pain, and antinociception: current concepts. Z Rheumatol 60(6):404-415.
- Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD (1997) Capsaicin-induced sensitization of primate spinothalamic tract cells is prevented by a protein kinase C inhibitor. Brain Res 772(1-2):82-86.
- Woolf CJ, Thompson SW (1991) The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 44(3):293-299.
- Woolf CJ (1996) Windup and central sensitization are not equivalent. Pain 66(2-3):105-108.
- Woolf CJ (1993) The pathophysiology of peripheral neuropathic pain–abnormal peripheral input and abnormal central processing. Acta Neurochir Suppl (Wien) 58:125-130.
- Woolf CJ, Thompson SW, King AE (1988) Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J Physiol (Paris) 83(3):255-266.
- Zou X, Lin Q, Willis WD (2002) Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience 115(3):775-786.
- Ganguly NK, Kaur T (1996) Mechanism of action of cholera toxin & other toxins. Indian J Med Res 104:28-37.
- Caudle RM, Mannes AJ, Keller J, Perez FM, Suckow SK, Neubert JK (2007) Sensitization of spinal cord nociceptive neurons with a conjugate of substance P and cholera toxin. BMC Neurosci 8:30.
