Somasundaram et al. C-terminal phosphorylation regulates the kinetics of a subset of melanopsin-mediated behaviors in mice. PNAS (2017), pii: 201611893. PMID: 28223508
Chronotype (morningness-eveningness) in mammals likely has multiple causes. Phase angle of entrainment is defined as the relationship between the timing of the biological clock and the timing of an external time cue, and if the primary explanation for morningness-eveningness is circadian, then a difference in phase angle of entrainment to the light-dark cycle would be expected between the two types. Intrinsically photosensitive retinal ganglion cells (ipRGCs) respond to light via both rod/cone-driven synaptic input and intrinsic melanopsin-based phototransduction; however, these two pathways are not functionally redundant. Melanopsin-based phototransduction in ipRGCs is critical for the regulation of the pupillary light reflex (PLR), circadian light responses, masking, and sleep. Therefore, understanding the kinetics and shutoff mechanisms for the melanopsin-based photoresponse will reveal how it contributes to a myriad of behaviors that are controlled by ipRGCs. Here, the authors show that the melanopsin photoresponse shutoff due to C-terminal phosphorylation determines the kinetics of the intrinsic light response in ipRGCs, the PLR, and reentrainment, but not masking and phase angle of entrainment. These results highlight the elaborate control of how the melanopsin photoresponse regulates vastly different light-mediated behaviors.
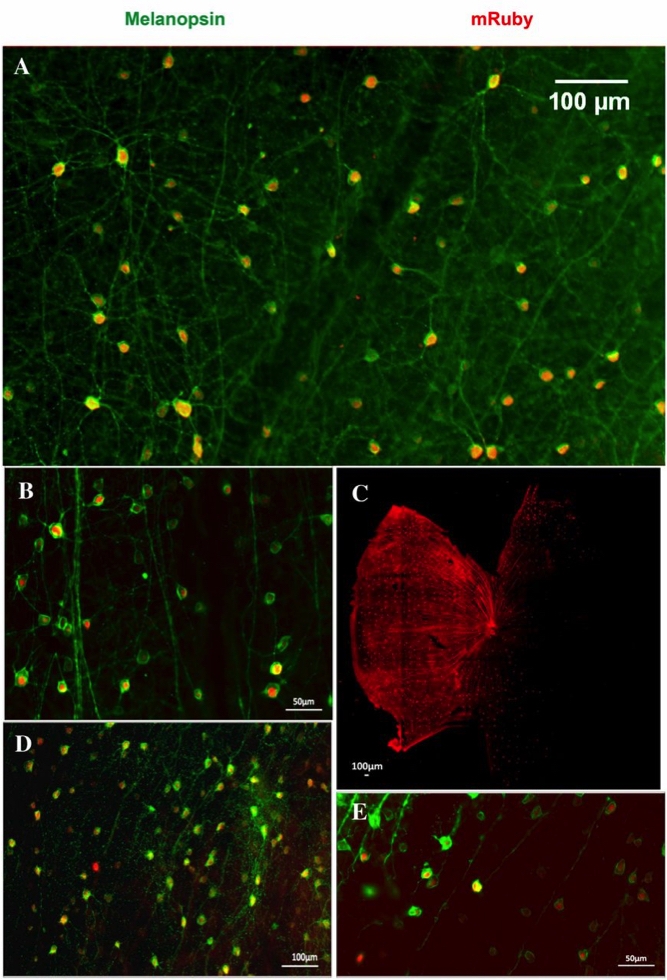
Immunofluorescence was performed using rabbit antimelanopsin (1:1,000, Cat.# AB-N38; Advanced Targeting Systems) as the primary antibody with a 2-d incubation period, followed by goat anti-rabbit IgG 488 as the secondary antibody. After immunostaining, the retinas were mounted and imaged using a Zeiss Axioimager M1 microscope. The images were processed in ImageJ.
A surprising outcome of this study is that in CPφ animals (mutant with no C-term phosphorylation sites), even after 16 h of light stimulation, the phase angle of entrainment was similar and masking responses were indistinguishable from masking responses in animals expressing WT melanopsin. This study has just scratched the surface for the complexity of the shutoff responses in ipRGCs. However, the authors have demonstrated that light-activated phosphorylation of melanopsin’s C terminus is an important post-translational modification regulating the lifetime of active melanopsin for regulating the PLR and the speed of reentrainment.
Questions? Ask a Product Manager
